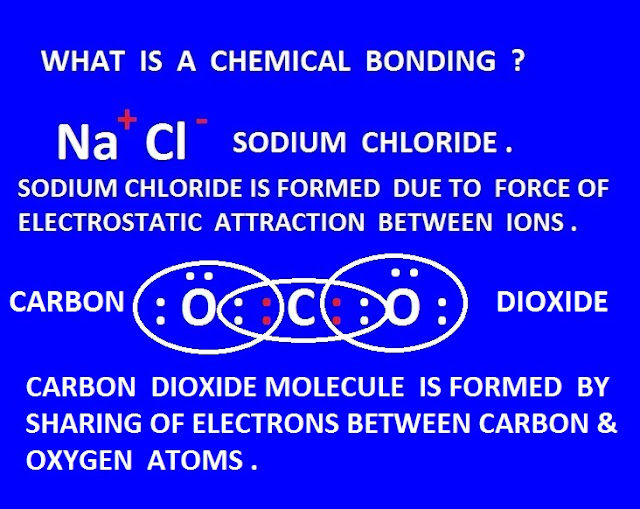
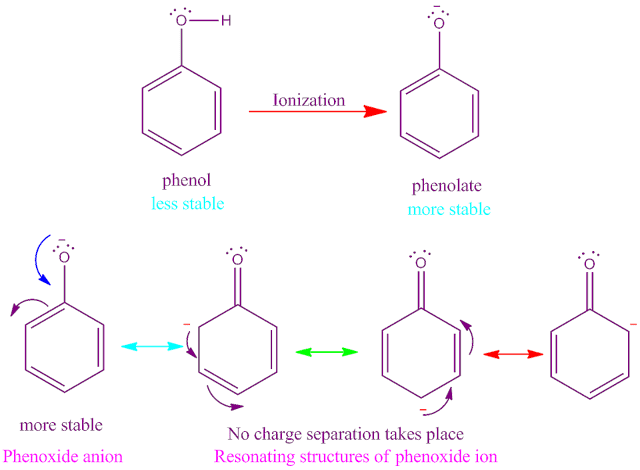
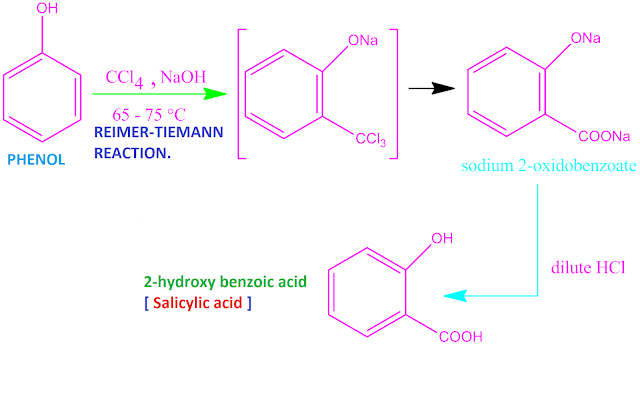
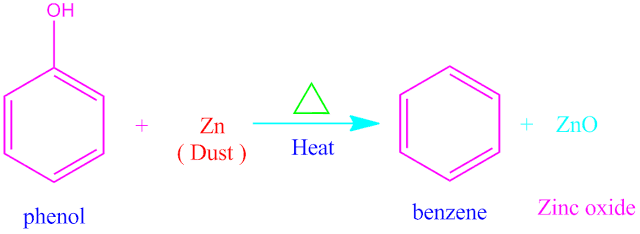
Covalent bond definition in chemistry
When a
chemical bond is formed between two similar or different atoms by
sharing of one or more electrons pairs by them to gain the more stable nearest
inert gas electronic configuration , then the bond is called covalent bond . It
is also called molecular bond.