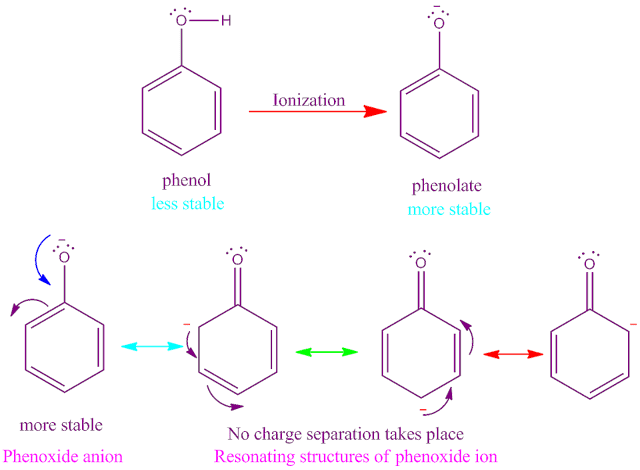
Why phenol is acidic in nature ?
The acidity of phenols arises due to its ability to lose proton to
form phenoxide ions. In case of phenol
molecule, the –OH group is directly attached to the sp2 hybridized
carbon atom of aromatic phenyl ring .
Due to – R effect of phenyl ring , the
lone pair electron on ‘O’-atom takes
part in the resonance with the pi
electron of benzene ring .
As a result , the oxygen atom gets partially
positive charge . Hence , oxygen atom attract the O – H bonded electron towards itself .
Consequently, O –H bond becomes more polar and easily break to release proton in aqueous solution along
with phenoxide anion .
Now this phenoxide ion formed is
stabilized by the delocalization of negative charge due to the resonance with
the pi electron of benzene ring.
It has been experimentally found that phenoxide
ion has greater stability than phenols .
Because in case of phenol charge separation takes
place during resonance. But in case of phenoxide anion no such charge separation
take place .
For the above two reason phenol is
acidic in nature . The acidic properties of phenol is also supported by its pKa
value .
The pKa value of phenol molecule is 9.98
. That is phenol is weak acid .
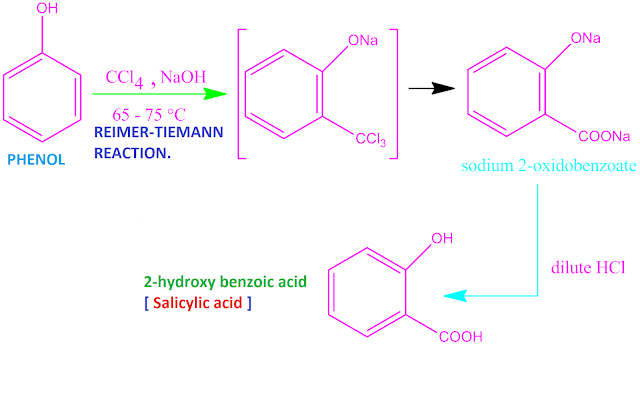
Phenol to salicylic acid change
Phenol can
be changed into salicylic acid by Reimer-Tiemann reaction . This is a chemical
reaction in organic chemistry by which ortho hydroxyl benzaldehyde as well as
ortho hydroxyl benzoic acid (salicylic
acid ) is obtained from phenol .
When the
reaction is carried out in presence of
chloroform and NaOH , the product
is ortho hydroxyl benzaldehyde .
But if this
reaction is carried out in presence of
carbon tetra chloride and NaOH base under 338 K – 348 K temperature , the product
is ortho hydroxyl benzoic acid or salicylic acid .
In first
step , the inter mediate product is
sodium 2-oxidobenzoate which on
hydrolysis by dilute HCl , the expected
salicylic acid is produced . The
reaction is shown below .
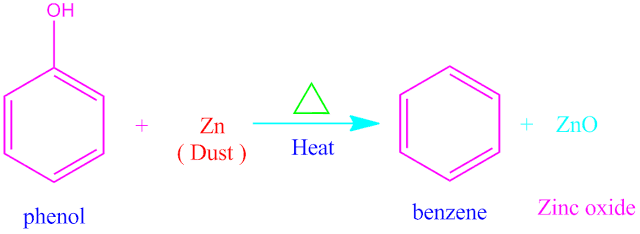
Phenol to benzene change
Phenol can be changed into benzene by
reduction . When phenol is heated in the presence of strong reducing agent , it
changed into benzene .
Zinc metal ( dust ) is used as reducing agent . In this
reaction , phenol reduced by Zn-metal and gets converted into benzene along
with ZnO as side product
The reaction of phenol to benzene changed is shown
below .
Summary :
Why phenol is acidic in nature ?
Phenol to salicylic acid change
Phenol to benzene change










No comments:
Post a Comment