Benzoic acid boiling point explanation.
Oxygen is more electronegative than hydrogen. So, O – H bond of benzoic acid is polar in nature .
As a result, benzoic acid forms inter-molecular hydrogen bond. To break this large number
of inter-molecular hydrogen bond , a great amount energy is required.
Consequently,
the boiling point of benzoic acid is high, that is, the boiling point of
benzoic acid is 249ᵒC .
Another
explanation of high boiling point of benzoic acid is molecular mass. The
molecular mass of benzoic acid is 122.04.
With
increasing molecular mass , the Vander waal’s force of attraction among the
molecules increases .Hence the
boiling point of benzoic acid becomes high.
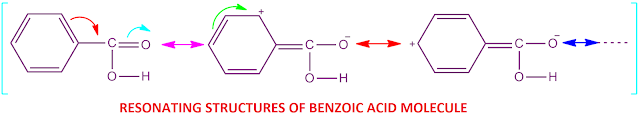
Benzoic acid strength explanation
In case of benzoic acid,the carboxylic acid group ( –COOH )
is directly attached to the sp2 hybridized carbon atom of aromatic benzene ring
that is, attached with phenyl group.
Now, +R effect of
phenyl group is greater than –I effect ( +R > –I ) . As a result of this ,
the O–H bond of benzoic acid becomes less polar than its expected value.
Consequently, the
tendency of benzoic acid to donate proton in aqueous medium decreases. For this
reason benzoic acid behave as a weak acid.
But ,the presence of electron
withdrawing group with benzene ring increase the acidic strength and presence
of electron repulsive group decrease the acidic strength of benzoic acid.
For example, p-nitro benzoic acid is more acidic than benzoic acid while p-methoxy benzoic acid is
less acidic than benzoic acid.
Why benzoic acid is more acidic than acetic acid?
In case of benzoic acid, the carboxylic acid group ( –COOH )
is directly attached to the sp2 hybridized carbon atom of aromatic benzene ring
, that is, attached with phenyl group.
Now, +R effect of phenyl group is greater than –I effect ( +R
> –I ). As a result of
this, the O–H bond of benzoic acid becomes less
polar than its expected value.
Consequently, the tendency of benzoic acid to donate proton
in aqueous medium decreases.For the above reason benzoic acid behave as a weak acid.
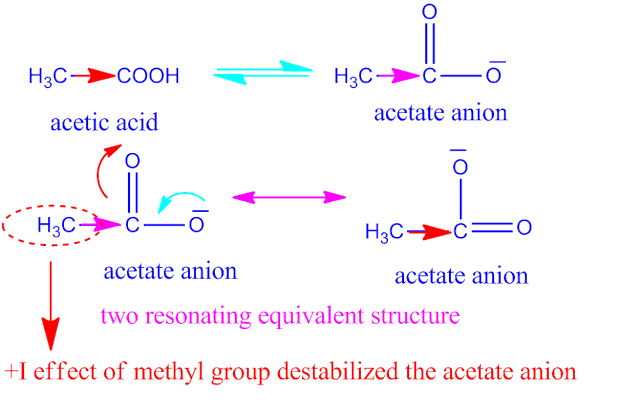
Although benzoic acid is more acidic than acetic acid.
Because, in case of acetic, the carboxylic acid group (–COOH ) is directly attached
to the methyl group ( –CH3 ) which have +I effect.
Now, the +I effect of methyl group is more effective than the
combined effect due to +R and –I effect
of phenyl group .Therefore, benzoic
acid becomes more acidic than acetic acid.
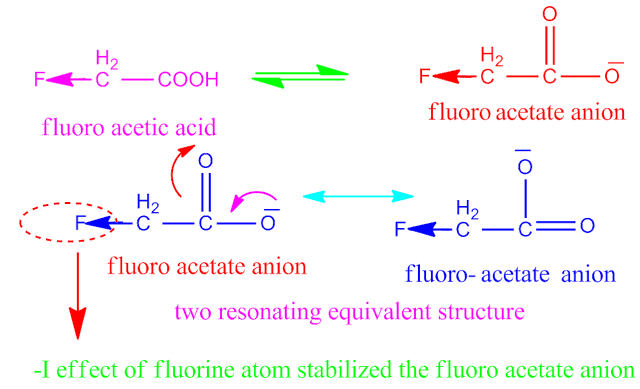
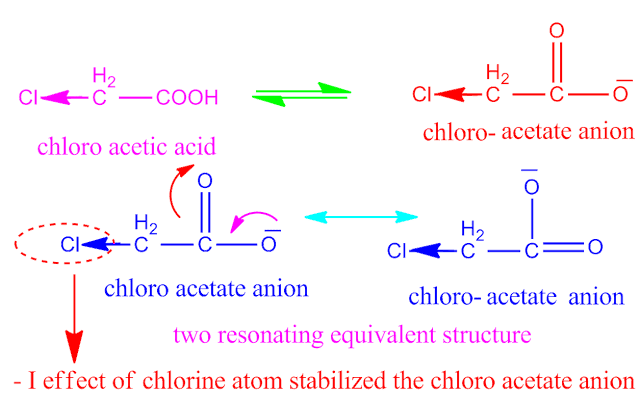
Which is more acidic fluoro acetic acid or chloro acetic acid?
The strength of any acid depends on the stability
of conjugate base of that acid .
Now, on
ionization of both fluoro acetic acid and chloro acetic acid in aqueous
solution, gives the conjugate base ,
fluoro acetate and chloro acetate anion respectively.
Both the
anion have two equivalent resonating structure . But,fluoro acetate anion becomes more stable than chloro acetate anion.
Because ,
fluorine is more electronegative than chlorine atom . That is why fluorine
have greater – I effect than that of
chlorine.
Since, fluoro acetate anion is more stable than chloro acetate anion , hence fluoro acetic acid is more stronger acid than chloro acetic acid.
Summary:
Benzoic acid boiling point explanation.
Benzoic acid strength explanation
Which is more acidic fluoro acetic acid or chloro acetic acid?
Why benzoic acid is more acidic than acetic acid?












Thank you for sharing that great information. Also, find about Acetic Acid Supplier who offers the best quality Acetic Acid In Bulk.
ReplyDelete