Covalent bond definition in chemistry
When a
chemical bond is formed between two similar or different atoms by
sharing of one or more electrons pairs by them to gain the more stable nearest
inert gas electronic configuration , then the bond is called covalent bond . It
is also called molecular bond.
In the
formation of covalent bond, the
atoms form the electron-pair in such a
way , so that a stable balance of attractive and repulsive forces acts among
the concern atoms.
What is covalency in chemistry ?
When two or
more atoms in their ground state or excited state , [ to attain the more stable
nearest inert gas electronic configuration ], by sharing of their outer most
valence electrons forms one or more electron pair, through which they gain a power for chemically attachment,
then this power of attachment is called covalency.
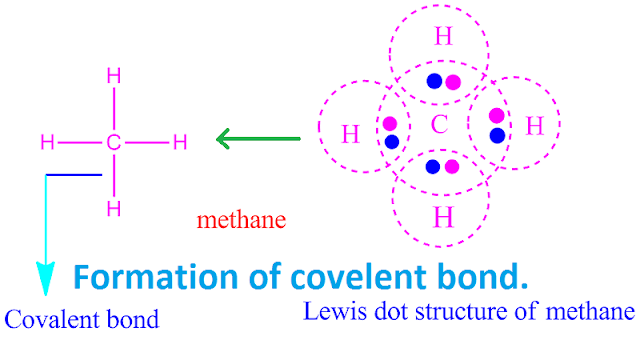
Covalent bond examples
There are a
large number of covalent compounds as
well as covalent bond in the world .
For example
, covalent molecule of elements and
covalent molecules of compounds .
A few
examples of both types of covalent bonded molecules and their formation are shown here.
Covalent bond formation energy
Any covalent
bond is formed by sharing of two opposite
spinning electrons.
Due to formation of electron pair with this two
opposite spinning electrons, a electro-magnetic force of attraction is appeared, this is called
covalent bond formation energy.
Types of covalent bond with definition and examples
There are
three types of covalent bond in chemistry .
( I ) covalent single bond
(
II ) covalent double bond and
( III ) covalent triple bond .
( I ) covalent single bond
If one electron-pair is equally shared between two
similar or different atoms , then the resulting bond formed is called covalent
single bond.
The bond is expressed by single dash . For example , Cl – Cl .
Formation of
covalent single bond by sharing of electron is shown below.
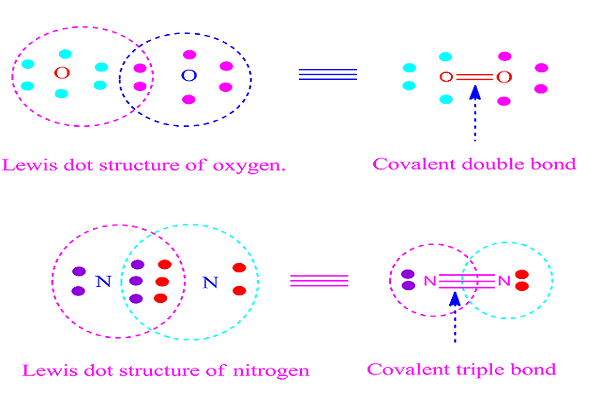
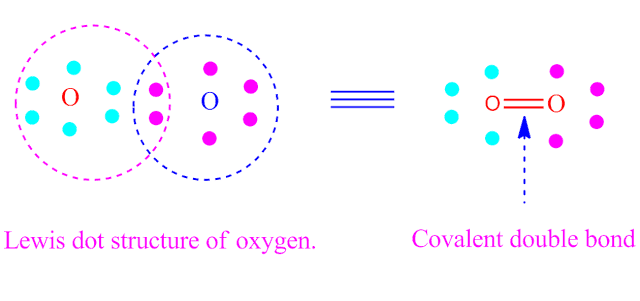
( II ) covalent double bond
If two
electron-pair is equally shared between two similar or different atoms , then
the resulting bond formed is called covalent double bond.
The bond is
expressed by double dash. For example, O = O.
Formation of
covalent double bond by sharing of electron is shown below.
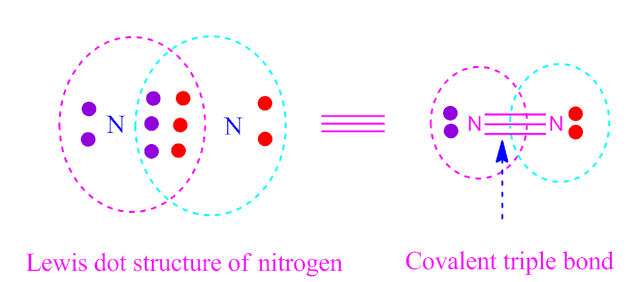
( III ) covalent triple bond
If three electron-pair is equally shared between twoatoms, then the resulting bond formed is called covalent triple bond .
The
bond is expressed by triple dash.For example
, nitrogen ( N2 ).
Formation of
covalent triple bond by sharing of electron is shown below.
Condition for the formation of covalent bond as well as chemical compounds .
Generally,
a covalent bond is formed by sharing of
electrons between two non-metallic atoms.
The number
of valence electrons of participating two atoms in covalent bonding must
be
4,5,6 and 7 ,so that each atom can complete their octet by sharing of electrons
and gain more stable inert gas electronic configuration.
The
ionization energy of the participating atoms in covalent bonding should be high .
The two
participating atoms in covalent bonding
should have high as well as equal
electron affinity.
The
electronegativity of the participating atoms in covalent bonding should be
equal or about equal. That is, their
electronegativity difference must be sufficiently low.
With
increasing the nuclear charge on the participating atom in covalent bonding,
the tendency for the formation of covalent bond increases.
Summary
- Covalent bond definition in chemistry.
- Covalent bond definition in chemistry.
- Covalent bond examples in chemistry .
- Covalent bond formation energy .
- Types of covalent bond with definition and examples.
- Condition for the formation of covalent bond as well as chemical compounds.












No comments:
Post a Comment