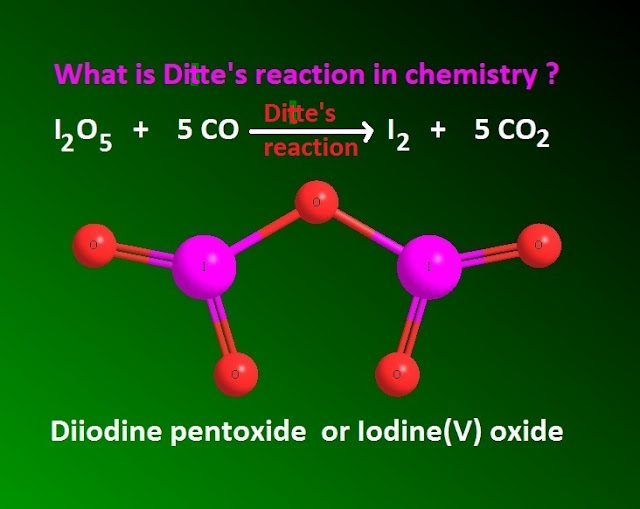
Ditte’s reaction in halogen chemistry
I2 O5 is a powerful oxidizing agent, which oxidize CO molecule in quantitative analysis to convert CO2 molecule along with molecular iodine .
The quantitative titration of this I2 by standard sodium thiosulfate (Na2S2O3) solution , using starch indicator , the quantity of CO can be measured .
The reaction
of I2 O5 with carbon monoxide ( CO ) , is known a Ditte’s reaction's .
Ditte’s reaction application .
This
reaction is used to measure the little amount of CO in air or any other
gaseous mixture .
This
reaction is so sensitive that it can determine one portion of CO in 30000
portion of air .
What is Deacon’s process ?
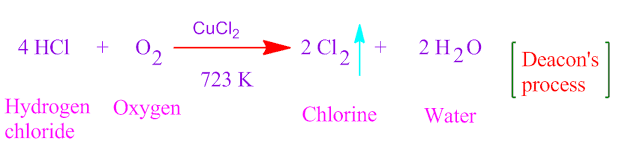
The chemical process , through which hydrogen chloride gas is oxidized by air oxygen in the presence of cupric chloride catalyst , under 723 K temperature, is known as Deacon’s reaction .
In this process hydrogen chloride gas is oxidized by air oxygen to produced chlorine gas . That is Deacon’s process is used to prepare chlorine gas from HCl gas .
Why ClF3 exist but FCl3 does not exist ?
The
electronic configuration of fluorine and bromine atom is shown below
respectively .
F –atom
, 1s2, 2s2, 2p5 .
Cl –atom ,
1s2 , 2s2, 2p6 , 3s2 , 3p5 .
From the
above electronic configuration , it is found that chlorine atom has vacant d-orbital in its valence shell .
As a result
, chlorine atom can expand its octet by using vacant d-orbital and easily exhibits +3 oxidation
state . For this reason ClF3
can exist .
On the other
hand , fluorine atom have no vacant
d-orbital in its valence shell. Due to absent of d-orbital , fluorine can
not expand its octet .
Consequently
, FCl3 does not exist . Besides
, being very strong electronegative , fluorine
can not show +3 oxidation state .
Oxoacid of fluorine
Generally fluorine does not form oxoacid . The most well known oxoacid of fluorine is HOF .
Although HOF is a very unstable
molecule . It is dissociate very easily giving HF and oxygen molecule .
Again
HOF does not form any salt or does not produced H + ion . These
experiment does not support the positive oxidation state of fluorine in HOF .
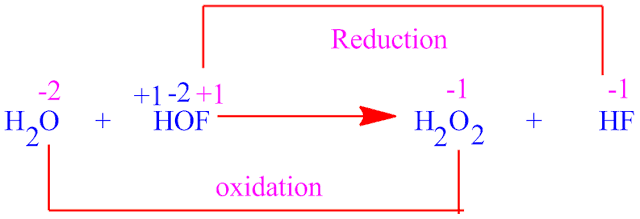
Yet, HOF oxidize H2O to H2O2 .This reaction certainly support the +1 oxidation state of fluorine in HOF
molecule . So it has been taking +1 oxidation state of fluorine in HOF molecule
.
Summary :
What is Ditte's reaction in halogen chemistry ?
Ditte's reaction application .
What is Deacon's reaction ?
Oxoacid of fluorine is very unstable ,why ?

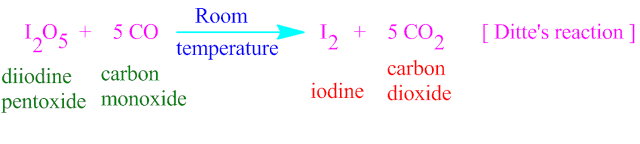







No comments:
Post a Comment