What is Dow process ? Give example .
When the
mixture of chloro benzene and 6 – 8 % solution of sodium hydroxide is heated at 350 ᵒC temperature under 300 atmosphere pressure , sodium
phenoxide is obtained .
Now, on acidic hydrolysis of sodium phenoxide , gives phenol . This process of preparation of phenol from chloro benzene is known as Dow process .
Now, on acidic hydrolysis of sodium phenoxide , gives phenol . This process of preparation of phenol from chloro benzene is known as Dow process .
This is an example of aromatic nucleophilic substitution reaction .
The reaction is carried out through active benzyne inter mediate formation .
Example of
Dow process is as follows,
Why the hydrolysis of o-nitro chloro benzene occurs easily than chloro benzene in basic medium ?
The hydrolysis of o-nitro chloro benzene and chloro benzene is an example of nucleophilic substitution reaction .
The nucleophilic substitution reaction is facilitated ,if the compound contain electron withdrawing group.
Now, in ortho nitro chloro benzene , the –NO2
group is an electron withdrawing group.
Due to – R effect of –NO2 group , the density of electron of benzene nucleus decreases .
As a result , the nucleophile can attack the targeted carbon atom easily .
Due to – R effect of –NO2 group , the density of electron of benzene nucleus decreases .
As a result , the nucleophile can attack the targeted carbon atom easily .
Besides, the
inter mediate carbanions produced , gains extra stability through resonance
with the π electrons of benzene ring and –NO2 group .
Consequently, the hydrolysis of o-nitro chloro benzene occurs easily than chloro benzene in basic medium .
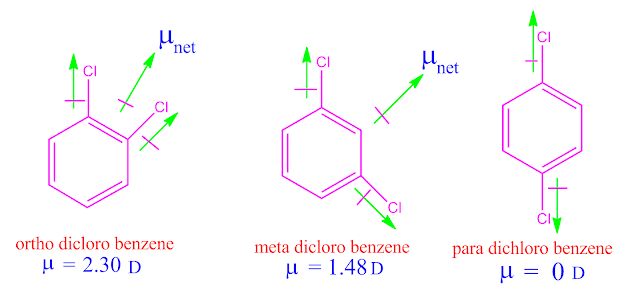
Compare the boiling point of ortho, meta and para dichloro benzene .
The boiling point of ortho, meta and para dichloro benzene are close to each other .
But ,among this three isomers the boiling point ortho isomer is greater than meta and para isomers .
This can be explained with their dipole moment value ,that is force of dipole –dipole attraction between the molecules .
The
experimentally dipole moment value of ortho, meta and para dichloro benzene are
2.30 D , 1.48 D and 0 D respectively.
So, the boiling point of the above three isomers should be in the following order,
So, the boiling point of the above three isomers should be in the following order,
o-dichloro
benzene > m-dichloro benzene > p-dichloro brenzene .
But the actual order is o-dichloro benzene > p-dichloro brenzene > m-dichloro benzene.
That is ,the boiling point ofpara isomer is slightly higher than meta isomer . Although the dipole moment of para dichloro benzene is 0 D .
Because the
p-dichloro brenzene molecules is more symmetrical . So , in crystal lattice of
p-dichloro brenzene , the molecules are close to each other .
Hence, to separate the molecules , high energy is required .
Hence, to separate the molecules , high energy is required .
Consequently,
the boiling point of para isomer is slightly higher than meta isomer.
Fluoro-arene
or aryl fluoride can not prepared by direct fluorination of aromatic
hydrocarbons, because fluorine is highly reactive in chemical reaction .
On reaction between fluorine and aromatic hydrocarbon an explosion occurs which can not be controlled .
On reaction between fluorine and aromatic hydrocarbon an explosion occurs which can not be controlled .
What is the best method for the preparation fluoro arene or aryl fluoride ?
The best
method for the preparation fluoro arene or aryl fluoride is known as shimaan reaction .
In this reaction , fluoro boric acid is added to the solution of benzene diazonium chloride salt .
In this reaction , fluoro boric acid is added to the solution of benzene diazonium chloride salt .
As a
result , a precipitate of insoluble diazonium fluoro borate is appeared . It is
dried after separating this precipitate from the solution .
Then ,on heating this dry sample gives fluoro arene or aryl fluoride .
Then ,on heating this dry sample gives fluoro arene or aryl fluoride .
What is Dow process ? Give example .
Why the
hydrolysis of o-nitro chloro benzene occurs easily than chloro benzene in basic
medium ?
Compare the
boiling point of ortho, meta and para dichloro benzene .
Why fluoro
arene or aryl fluoride can not prepared by direct fluorination of aromatic
hydrocarbons ?
What is the
best method for the preparation fluoro arene or aryl fluoride ?













Take a look at healthy region
ReplyDelete