What is
Cannizzaro reaction?
Aldehydes that do not have an
alpha hydrogen atom undergo auto-oxidation reduction reactions when heated with
concentrated or 50% NaOH or KOH solutions.
That is, half of the
participating aldehydes molecules are oxidized to carboxylic acids (as sodium
or potassium salt) and half are oxidized to alcohols. This
auto-oxidation-reduction reaction is called Cannizzaro reaction. Cannizzaro
reaction is also called disproportionation reaction.
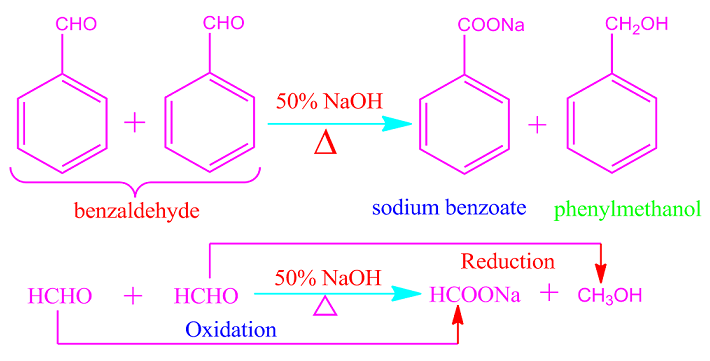
Formaldehyde, trimethylacetaldehyde, benzaldehyde or any other aromatic aldehydes participates in the Cannizzaro reaction because of the absence of alpha hydrogen.
For example, when formaldehyde is heated with 50% NaOH solution, one molecule of the participating formaldehyde is oxidized to the sodium salt of formic acid and other molecule is reduced to methanol.
In the similar way, when
benzaldehyde is heated with 50% NaOH solution, one molecule of the
participating benzaldehyde is oxidized to the sodium salt of benzoic acid and
other molecule is reduced to benzyl alcohol.
Mechanism of Cannizzaro
reaction
The Cannizzaro reaction is essentially a unidirectional reaction. The Cannizzaro reaction proceeds in three steps.
Step-I: The
nucleophile that is, OH- ion, present in the reaction medium attacks the
carbonyl carbon of the aldehyde to form the anion.
Step-II: In second step, the resulting anion causes the transfer of a hydride ion to the carbonyl carbon of another aldehyde. This results in the formation of one molecule carboxylic acid and one molecule an alkoxide ion.
Step-III: In the third step, proton exchange between
carboxylic acid and alkoxide ion produces carboxylate ion and alcohol.
What is crossed Cannizzaro reaction?
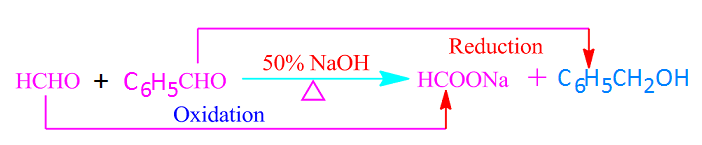
When two different aldehydes
without alpha hydrogen atoms react with each other in the presence of 50% NaOH
or KOH, the reaction is called a crossed Cannizzaro reaction.
Similar to the Cannizzaro reaction, in the crossed Cannizzaro reaction, one molecule of the aldehyde is oxidized to a carboxylic acid and one molecule is reduced to an alcohol.
In this case, among the two aldehydes, the aldehyde whose carbonyl carbon is more reactive is oxidized. For example, in the crossed Cannizzaro reaction between HCHO and C6H5CHO, HCHO is oxidized easily to formic acid.
Because of, among formaldehyde and benzaldehyde, the carbonyl carbon of formaldehyde is more reactive.
What is intra-molecular Cannizzaro reaction?
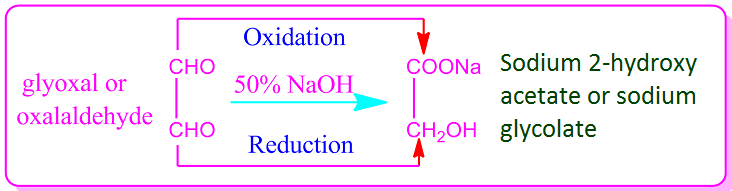
In general, all aldehydes in
which two aldehyde groups are present show intra-molecular Cannizzaro
reactions. For example, heating of 1,2-dialdehyde or glyoxal with 50% NaOH or
KOH results in an intra-molecular Cannizzaro reaction.
That is, in this case, one of
the two aldehyde groups of dialdehyde is oxidized and the other is reduced to
produce the sodium or potassium salt of glycolic acid.
What are the limitations or drawbacks of Cannizzaro reaction?
(I)Chloral does not
participate in the Cannizzaro reaction in the presence of alkali, although it
has no alpha-hydrogen atom. Because, heating of chloral with concentrated NaOH
breaks its C–C bond to form CHCl3 and HCOONa.
(II) Despite having an
alpha-hydrogen atom of isobutyraldehyde or 2-methyl propanal, it participates
in the Cannizzaro reaction in the presence of strong alkaline solutions.
A possible reason for this is that the corresponding carbanion is not stable enough due to the presence of two methyl groups on the alpha carbon of the compound. So this aldehyde can undergo aldol condensation reaction on one side and Cannizzaro reaction on the other side.
(III)Aldehyde containing
alpha-hydrogen is heated with a strong alkali solution to form a brown resin in
a poly-condensation reaction.
Cannizzaro reaction, crossed Cannizzaro reaction, intra-molecular Cannizzaro reaction, limitations or drawbacks of Cannizzaro reaction,
- What is Cannizzaro reaction?
- What is crossed Cannizzaro reaction?
- What
is intra-molecular Cannizzaro reaction?
- What are the limitations or drawbacks of Cannizzaro reaction?










No comments:
Post a Comment