Why is phenol more acidic than aliphatic alcohols?
Phenol
is acidic in nature. Because of phenol can donate proton in aqueous solution.
Although, phenol is weakly acidic but
phenol is more acidic than aliphatic alcohols.
The
strength of acid depends on the tendency of donating proton in aqueous solution
easily. It has been experimentally found that phenol can donate proton easily
than aliphatic alcohols.
Consequently
phenol becomes more acidic than
aliphatic alcohols. The acidic properties of phenol can be explained on the
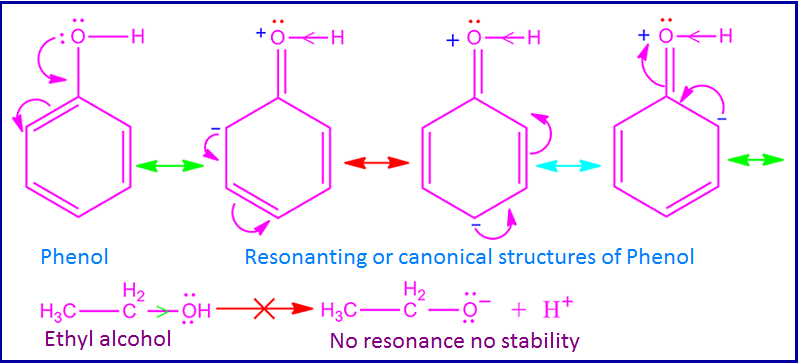
basis of its resonance. The lone pair of electron on oxygen atom takes part in
resonance with the π electron of
benzene ring.
As
a result, oxygen atom gets partial positive charge and hence it attracts O – H bonded electron towards itself.
Consequently O – H bond become polar
as well as weak and release H+ ion easily.
But in case of aliphatic alcohols no such resonance takes place. Hence O – H bond of aliphatic alcohols become stronger than the O – H bond of phenol. Therefore aliphatic alcohols have a very less tendency to donate H+ ion. That is, phenol becomes more acidic than aliphatic alcohols.
It
has been experimentally found that phenol is about one million times more
acidic than aliphatic alcohols. Phenol reacts with active metals like sodium or
potassium and sodium or potassium phenoxide along with hydrogen gas.
Besides
of being more acidic than aliphatic alcohol phenol reacts with caustic soda or
sodium hydroxide forms sodium phenoxide salt and water. But aliphatic alcohols
can’t react with metallic sodium or sodium hydroxide.
Phenol
(pKa = 8-10) can turn blue litmus into red, but aliphatic alcohols (pKa = 16–20)
cannot turn blue litmus into red.
Why is phenol more acidic than ethyl alcohol?
Phenol is more acidic than ethyl alcohol. This can be explained on the basis of resonance effect and inductive effect of phenol and ethyl alcohol. The – OH group of phenol has +I effect and also +R effect. But in case of phenol +R effect dominates over +I effect.
Due
to this +R effect, the lone of electron on oxygen atom takes part in the
resonance with π electron of benzene ring. As a result, oxygen atom of phenol
gets partial positive charge.
Consequently, oxygen atom attracts O – H bonded electron towards itself and hence O – H bond polarity increases. For this reason, O – H bond breaks easily and phenol donate proton (H+) in aqueous solution.
Ethyl alcohol, on the other hand, does not
participate in any such resonance like phenol. Again the +I effect of the ethyl group reduces the polarity of the
O – H bond.
As a result, the O – H bond of ethyl
alcohol is stronger than the O – H bond of phenol. As a result, the tendency of ethyl alcohol to donate protons is
reduced.
Consequently
ethyl alcohol is less
acidic than phenol.
- Why is phenol more acidic than aliphatic alcohols?
- Why is phenol more acidic than ethyl alcohol?
- Why is phenol more acidic than methyl alcohol?
Phenol more acidic than aliphatic alcohols, phenol more acidic than ethyl alcohol, phenol more acidic than methyl alcohol,









No comments:
Post a Comment