Balancing equations in chemistry by ion electron method for class 11
The balancing of equations of any chemical reactions are very important matters in chemistry.
This
is necessary, because of law of mass action says that the total mass of
reactants before is equal to the total mass of products after reaction.
Now,
most of the chemical reactions are oxidation-reduction reaction and they have a
chemical equation.
The
equations of this oxidation-reduction reaction can be balanced with two methods. The one of this two is ion-electron
method and the other is oxidation number method.
Balancing
equations rules ion-electron method
Scientists Jatle and Lamer introduced the
ion-electron method for balancing equations. This procedure is done in roughly
eight steps.
Step1. If the reaction equation is written in
molecular form, then the equation must be written in ionic form.
Step2.
The reaction is divided into two half reactions with the help of ions and
electrons. One is a half-oxidation reaction and the other is a half-reduction
reaction.
Step3. When writing the oxidation reaction,
the reducing agent is written to the left of the arrow and the oxidized substance to the right.
In the case of oxidation reaction, oxidant is written to
the left of the arrow and reducing substance is written to the right.
Step4.
The number of electrons is written to the right of the arrow to denote the
exclusion of electrons in the oxidation half reaction and to the left of the
arrow to indicate the acceptance of electrons in the reduction half reaction.
Step5.
Then each half reaction is balanced. A few steps are followed to balance this
two half reaction.
First,
in each half reaction, the number of atoms other than the H and O-atoms on
either side of the arrow is equalized.
When the reaction takes place in an acidic
solution, H2O or H + ions are used to equalize the number of H and O-atoms on
either side of the arrow.
First the number of O-atoms is equalized.
An H2O nucleus is added for each O-atom on the
side of the arrow that has the lowest number of O-atoms.
Two
H + ions are then added for each H2O atom on opposite sides to equalize the
number of H-atoms.
When
the reaction takes place in basic medium, H2O or OH-ions are used to equalize
the number of H and O-atoms on either side of the arrow.
Adding
an H2O molecule to the side of the arrow with the extra O-atom on the other
side will add two OH-ions.
If
the number of H-atoms is not equal even after the number of O-atoms is equal on
both sides of the arrow, then one OH-ion is added for each additional H-atom on
the side with the additional H-atom and one H2O molecule on the opposite side. But
no half-reaction will have H + or OH-ions at the same time.
Step6.
The charge is then equalized on both sides of each half reaction.
On the side where the negative charge is less,
the charge on both sides is equalized by adding electrons.
Step7.
To equalize the number of electrons in two half reaction, one or both of the
two reactions are multiplied by the smallest number required.
Step8.
Equilibrium equations are obtained by adding the two equilibrium half reaction
thus obtained and subtracting the common substances from both sides.
Balancing equations in chemistry simple examples,
Balancing
equations in acidic medium:
The
equations in the ion electron method of reactions occurring through acidic and
alkaline medium are shown below.
The reaction of oxalic acid with KMnO4 in the
presence of sulfuric acid is an example of reaction that occurs in acidic
medium.
In this case the molecular reaction of reaction, the ionic form of equation, the oxidation half-reaction and the reduction half-reaction are shown below.
Now
multiply the equation (1) by 5 and the equation (2) by 2 and after adding this
two we get the equation is,
This
equation is the balancing equation of the reaction in ionic form. If we express
the equation in molecular form we get,
If the number of K-atoms and sulfate radicals
on the left and right are equal, then we get,
So
the determinable equation is the balancing equation in the ion electron method.
Balancing equations in alkaline medium:
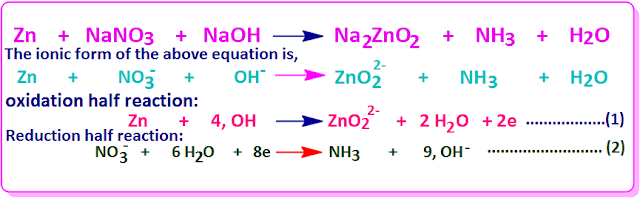
Again
the reaction of NaNO3 with Zn in the presence of NaOH base is an example of a
reaction occurring through the base.
In this case, the molecular form , the ionic form, oxidation half and reduction half reactions are shown below.
Now, after multiplying the equation (1) by 4, we add it to the equation 2 we get,
If we express the equation in molecular form
we get,
So the determinable equation is the balancing equation in the ion electron method.
- Balancing equations in chemistry by ion electron method for class 11
- Balancing equations by ion electron method simple examples
Balancing equations, balancing equations in chemistry,
balancing equations by ion electron method for class 11, balancing equations in
chemistry simple examples,
Read more : Acid-base neutralization reactions

















No comments:
Post a Comment