What is acid base neutralization reactions definition in chemistry?
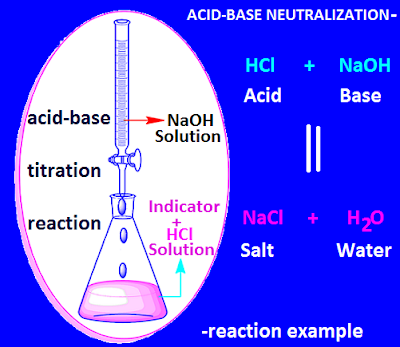
There are different types of chemical reactions in chemical science. Acid-base neutralization reaction is an example of one type of chemical reactions.
In
acid-base neutralization reaction, an acid reacts with base to produce salt and
water. At the end of the reaction, the solution overall becomes neutral.
The chemical process by which an equivalent amount of acid
reacts with an equivalent amount of alkali results in the complete
disappearance of acid and alkali properties, producing salt and water, that chemical process is called acid-base
neutralization reaction.
For example, NaCl and water are produced by the reaction of equivalent amount of NaOH with an equivalent amount of concentrated HCl.
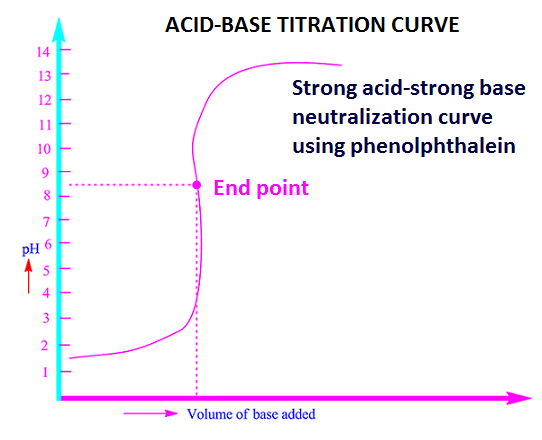
Acid-base neutralization curve of strong acid (HCl) and strong base (NaOH) are shown below.
Due
to the absence of excess H + and OH-ions present in the resulting solution, the
properties of acids and alkalis are completely lost.
What are the types of acid-base neutralization reactions?
There
are different types of acid-base neutralization reactions. Out of
these, four types of acid-base neutralization reactions are important. These
four types reaction are,
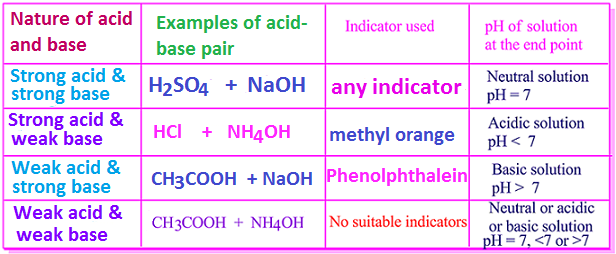
1. Acid-base neutralization reaction between strong acids and strong bases. For example, reaction between concentrated sulfuric acid and sodium hydroxide [Conc. H2SO4 + NaOH].
In this case any indicator is used to
indicate the end point or neutralization point.
2. Acid-base neutralization reaction between strong acids and weak bases. Such as reaction between concentrated hydrochloric acid and ammonium hydroxide [Conc. HCl + NH4OH].
In this case, methyl orange is used as an
indicator to indicate the end point or neutralization point.
3. Acid-base neutralization reaction between weak acids and strong bases. For example, reaction between acetic acid and sodium hydroxide [CH3COOH + NaOH].
In this case, phenolphthalein indicator is
used to indicate the end point or neutralization point.
4. Acid-base neutralization reaction between weak acids and weak bases. Such as reaction between acetic acid and ammonium hydroxide [CH3COOH + NH4OH].
In
this case there is no suitable indicator to indicate the neutralization point
or end point. Indicators
are used on the basis of acids and bases used in this case.
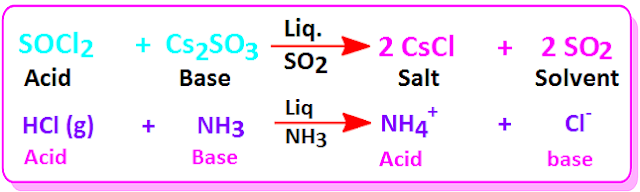
Acid
base neutralization reaction in non aqueous solvent
Acid-base neutralization reaction is also take place in
non aqueous solvent, such as liquid ammonia, liquid sulfur dioxide and liquid
HF acid.Few more examples of acid- base neutralization reactions in non aqueoussolvent are shown below.
What indicator is used in acid base neutralization reaction?
In the reaction of acids and alkalis, the moment at which
the properties of acid and alkali are completely lost and the solution isneutralized, that moment is called end point.
Different indicators are used to determine the end point of different
types of acid-base neutralization reactions.
A
list of indicators those used for different types of acid-base neutralization
reactions are shown below.
What are the uses of acid-base neutralization reactions?
Acid-base neutralization reactions determine the strength of an unknown amount of acid and alkali.
Simple stoichimetric calculations with the known volume of the unknown and the known volume and molarity of the added chemical gives the molarity of the unknown.
There
are many uses for acid-base neutralization reactions. One of the most common uses is antacids.
Antacids are used to neutralize the excess acid needed in the stomach and to raise the pH value of the digestive juices to the appropriate level. Usually antacids like Al (OH) 3, Mg (OH) 3, MgCO3, etc. are used.Blood pH is also maintained through acid-base neutralization reaction.
Another
common use of acid-base neutralization reactions is to control the pH of fertilizer
and soil. Calcium hydroxide or calcium carbonate may be worked into soil that
is too acidic for plant growth.
Fertilizers
that develop plant growth are made by neutralizing sulfuric acid or nitric acid
with ammonia gas, making ammonium sulfate or ammonium nitrate.
Besides,
the pH of wastewater is measured by the acid-base neutralization reaction. This
reduces water pollution in the environment. For pH control, popular chemicals
including CaCO3, CaO, Mg (OH)2, and NaHCO3.
The
selection of an appropriate neutralization chemical depends on the particular
application.
- What is acid-base neutralization reactions definition in chemistry?
- What is acid base neutralization reaction example?
- What are the types of acid-base neutralization reactions?
- What indicator is used in acid base neutralization reaction?
- What are the uses of acid-base neutralization reactions?
acid-base neutralization, neutralization of acid and bases, acid base neutralization
reactions, acid base neutralization reactions examples, acid base
neutralization equation, acid base neutralization reactions equations, acid
base neutralization reactions answers, acid base neutralization reaction is an
example of, acid base neutralization reaction experiment, acid base
neutralization is what type of reaction, acid base neutralization endothermic
or exothermic,
Read more : Oxidation agent definition with examples.









No comments:
Post a Comment