Co-ordinate covalent bond definition in chemistry
If one of the two participating atom in a chemical attachment, provide one pair of electrons and both they complete their octet by sharing this electron pair, then the
power of the two
participating atom for the attachment to each other, is called co-ordinate
covalency.
The chemical bond which is created due to co-ordinate covalency is called co-ordinate bond.The co-ordinate bond is also called dative bond.
Although
co-ordinate bond is also one type of covalent bond. But this bond is polar in
nature. Because, the
donor atom gets positive charge and the acceptor atom gets negative charge.
Like
covalent bond, co-ordinate bond is also produced by the overlapping of two
atomic orbital .
Since the
atomic orbital has a specific direction , hence co-ordinate bonds are also have
a specific direction.
What is co-ordinate covalency?
If one of the two participating atom in a chemical attachment, provide one pair of electrons and both they complete their octet by sharing this electron pair, then the
power of the two
participating atom for the attachment to each other, is called co-ordinate
covalency.
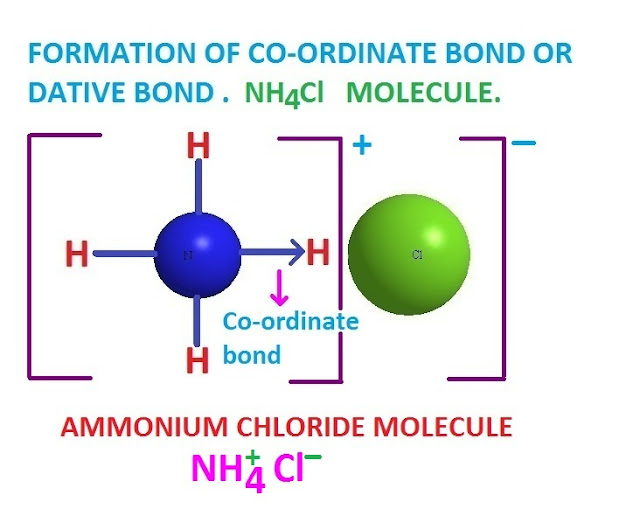
Co-ordinate covalent bond examples .
There are many examples of co-ordinate covalent bond or co-ordinate covalency .
Such as , NH3.BF3
, LiAlH4 , NaBH4, NH4Cl, Al2Cl6 etc compounds contain co-ordinate covalent
bond .
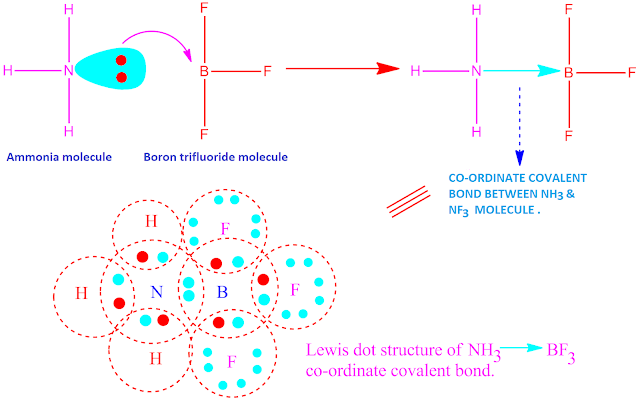
Formation of
co-ordinate covalent compound, like NH3.BF3
is shown below .
The nitrogen atom in ammonia have one lone pair of electrons . On the other hand, the boron atom in BF3 molecule have incomplete octet.So B-atom have a tendency to gain two electrons for completion of its octet.
Hence, in reaction between ammonia and boron tri-fluoride, ‘N’-atom of ammonia molecule acts as electron donor and ‘B’-atom in BF3 acts as electron acceptor.
But both they complete their octet by sharing of electron pair equally . In this way , NH3 and BF3 molecule form a co-ordinate covalent bond to each other, resulting in the formation an adduct compound.
Now, the molecules or ions which give up electron pair, they are called Lewis base and the molecules or ions which accept electron pair, are called Lewis acid. Hence, here NH3 acts as a Lewis base and BF3 acts as a Lewis acid.
Co-ordinate covalent bond formation conditions
( I ) Between the two participating atoms, the donor atom must have one lone pair of electron.
( II ) The
acceptor atom must have a vacant orbital
so that the atom can accept the lone pair of electrons.
( III ) The complete displacement of electrons
from donor atom to acceptor atom does not occur. Both the
atom ( donor and acceptor ) shall be use the electron pair equally.
Characteristics of co-ordinate covalent compounds .
Co-ordinate covalent bond is one type of covalent bond. So the characteristics of co-ordinate covalent compounds are similar to that of covalent bond .
( I )
Co-ordinate covalent compounds may be solid, liquid or gaseous in room
temperature .
( II ) Co-ordinate covalent compounds are
polar in nature. Actually Co-ordinate covalent bond is more polar than
covalent bond and less polar than ionic bond.
Consequently,
the melting and boiling point of Co-ordinate covalent compounds are higher than
covalent compounds but less than ionic compounds.
(III )
Co-ordinate covalent compounds have a co-ordinate covalent bond . But all other
bonds are covalent bond.
Hence Co-ordinate covalent compounds are soluble in non-polar solvent ( benzene ,
carbon disulfide, carbon tetrachloride etc )but the solubility in polar
solvent is very much less .
( IV ) Compounds
with co-ordinate covalent bond does not ionize in aqueous solution as well as
in melting condition.So they can not conduct electricity.
( V) Co-ordinate covalent bond is rigid and they have specific
direction . So co-ordinate covalent compounds show isomerism properties .
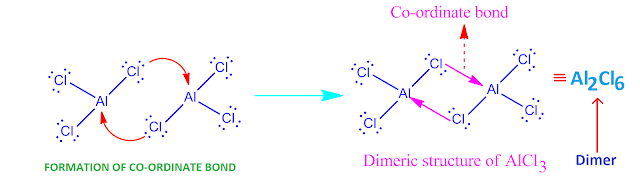
Why does AlCl3 exist as a diner ?
The chlorine
atom in AlCl3 molecule have three lone pair of electrons . On the
other hand, ‘Al’-atom have incomplete octet .
So ‘Al’-atom
have a tendency to gain two electrons for completion of its octet.
Hence, Cl-atom of one AlCl3 molecule donate electron to the Al-atom of
another AlCl3 molecule and form a co-ordinate bond.
But both they complete their octet by sharing of
electron pair equally . In this way , two AlCl3 molecule form a co-ordinate covalent bond to each other,
resulting in the formation of a dimer molecule Al2Cl6
.
Now, the
molecules or ions which give up electron pair , they are called Lewis base and
the molecules or ions which accept electron pair , are called Lewis acid.
Hence, here chlorine
atom acts as a Lewis base and ‘Al’-atom acts as a Lewis acid.
Why BCl3
trigonal planar but AlCl3 tetrahedral?
Boron atom in BCl3 molecule is sp2 hybridized. Three half filled hybridized orbital of ‘B’-atom is overlapping with the three half filled p-orbital of ‘Cl’-atom ,resulting in the formation of trigonal planar BCl3 molecule .
Boron atom
can not attach with four chlorine atom due to its small size .Hence, the
geometry of BCl3 molecule is trigonal planar.
On the other
hand, chlorine atom in AlCl3 molecule have three lone pair of electrons
and ‘Al’-atom have incomplete octet. So ‘Al’-atom
have a tendency to gain two electrons for completion of its octet.
Hence,
‘Cl’-atom of one AlCl3 molecule donate electron to the ‘Al’-atom of
another AlCl3 molecule.
In this way, two AlCl3 molecule
form a co-ordinate covalent bond to each
other, resulting in the formation of a dimer molecule Al2Cl6. For this
reason, the shape of AlCl3 molecule is tetrahedral.
Summary
- Co-ordinate covalent bond definition in chemistry
- What is co-ordinate covalency ?
- Co-ordinate covalent bond examples .
- Co-ordinate covalent bond formation conditions .
- Characteristics of co-ordinate covalent compounds .
- Why does AlCl3 exist as a diner ?
- Why BCl3 trigonal planar but AlCl3 tetrahedral ?









No comments:
Post a Comment