Complex compounds or co-ordination compounds definition in chemistry
There are a large number of molecular compounds which maintain their identities even when dissolved in water or any other solvents and their physical and chemical properties are completely different from those of the constituents, are called complex compounds or co-ordination compounds.
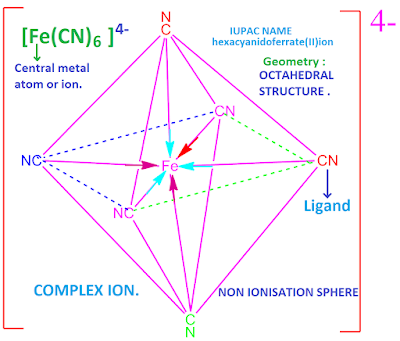
For example, potassium ferro-cyanide, K4 [ Fe ( CN ) 6 ] . Potassium ferro-cyanide is obtained by the reaction of Fe(CN )2 and KCN.
Besides this,
there are many other examples of complex compounds. Such as, [Co ( NH3 )6 ] Cl3 , [ Ni ( NH3
)6 ]Cl2 , [Pt ( NH3 )2 Cl4
] , K2[ Pt Cl4 ] , K2[ Hg I4 ] etc.
What is imperfect complex ion or complex compound?
Complex ions or co-ordination entity that can not maintain their independence existence in solution and they are dissociated into their constituents ions partially,
those
complex ions are called imperfect complex ion or complex compound.
For example,
potassium tetracyanido cadmate(II) with molecular formula, k2[Cd ( CN )4 ]. k2[Cd ( CN )4 ] is dissociated in solution into K + and [ Cd ( CN ) 4
] 2- ion.
Then, [ Cd ( CN ) 4 ] 2-
ion is further dissociated and released free central metal ion , Cd 2+ and Ligand CN – .
We can
proved it by passing H2S gas through this solution . If we passed H2S
gas through this solution, a yellow color precipitation of CdS is appeared which
proved the existence of free Cd 2+ ion in solution.
What is perfect complex ion or complex compound ?
Complex ions
or co-ordination entity that exhibit very much less degree of dissociation in
solution and hence maintain their independence existence in solution
completely,
those complex ions are called perfect complex ions or complex
compounds.
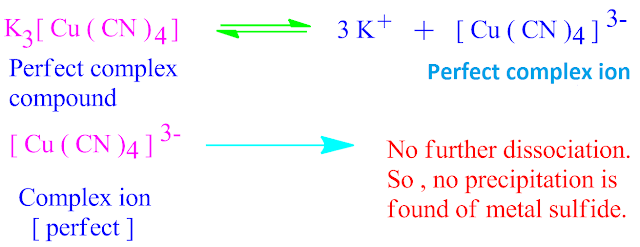
For example, K3[Cu ( CN )4 ]
complex compound is dissociated in solution into K + and [ Cu ( CN )4 ]3– complex
ion.
But if we passed H2S gas through this solution, no precipitation is found. This means that, [ Cu ( CN )4 ]3– complex ion remain unchanged in solution .That is, it is stable in nature.
Summary
- Complex compounds or co-ordination compounds definition in chemistry.
- Complex compounds or co-ordination compounds examples.
- What is perfect complex ion or complex compound ?
- What is imperfect complex ion or complex compound?










No comments:
Post a Comment