What is ammonium carbonate compound?
Ammonium carbonate is an inorganic
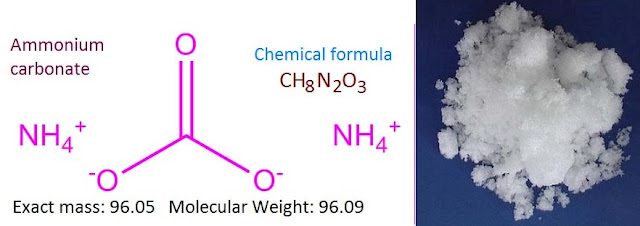
salt with molecular formula (NH4)2CO3. It is
also known as baker’s ammonia and was a precursor to the more modern leavening
agent baking soda and baking powder.
The molar mass of ammonium carbonate is 96.09 g/mol. It is a colorless crystalline solid or white powder with a strong odor of NH3 and has a sharp ammoniacal taste.
It is non-combustible and completely dissolves in water. The melting point of ammonium carbonate is 331K. But it decomposes to its boiling point.
Ammonium
carbonate is a salt of weak carbonic acid. When carbonic acid is reacts
with ammonia then ammonium carbonate
is obtained.
2
NH3 + H2CO3 → (NH4)2CO3
It
is also prepared by the sublimation of a mixture of ammonium sulfate and CaCO3
and occurs as a white solid powder.
Since,
ammonium carbonate is an inorganic
as well as ionic compound hence it is soluble in water. The density of ammonium carbonate is 1.5 g/cm3.
On
heating, ammonium carbonate readily
degrades to gaseous NH3 and CO2. For this reason, it is
used as a leavening agent and also as smelling salt.
Ammonium carbonate forms ammonium
salts and carbon dioxide when it reacts with acids. With bases, it produces
ammonia gases.
Uses of ammonium carbonate
Leavening
agent
Ammonium carbonate is commonly used
as a leavening agent in traditional recipes. It was the precursor to the more
modern leavening agent baking soda and baking powder.
Ammonium carbonate acts as a heat
activated leavening agent and it breaks down into CO2 (leavening),
ammonia and water.
It is sometimes combined with NaHCO3
to imitative as a double acting baking powder and to help mask any ammonia
smell not baked out.
Baker’s
ammonia should be used to create thin dry baked goods like crackers and
cookies. This allows the strong ammonia smell to bake out,
Other uses
Ammonium carbonate is the main
component of smelling salts, although the commercial scale of their production
is small.
Ammonium carbonate is used as an
active ingredient to prepare cough syrup which is used to help relieve symptoms
of bronchitis.
Ammonium carbonate is also used as an
emetic. It is also found in smokeless tobacco products like Skoal, and it is
used in aqueous solution as a photographic lens cleaning agent.
- What is ammonium carbonate compound?
- Uses of ammonium carbonate
- What is ammonium carbonate used for?
- What is baker’s ammonia in chemistry?
ammonium carbonate, uses of ammonium carbonate,
baker’s ammonia,










No comments:
Post a Comment