What is ionization energy of elements in chemistry?
The term ‘ionization
energy’ of elements is a very important topic in chemistry. We can know
some important properties of elements from the value or idea of ionization energy.
The ionization
energy or first ionization energy
of an element is defined as the amount of energy required to remove the most
loosely bounded valence electron from an isolated gaseous atom, converting it
into unique positive ion.
Since in this process heat energy is supplied to
remove electron hence it is an endothermic process.
Periodic variation of ionization energy
Variation of ionization energy along a period:
When we going from left to right along a period,
the nuclear charge of the elements increases, at the same time atomic radius of
elements decreases.
Hence the force of attraction of nucleus toward the outer most electron increases. So the amount of energy required to remove the outer most valence electron of the elements increases from left to right for a particular period. That is, ionization energy of elements increases from left to right along a period.
Variation of ionization
energy along a group:
When we move from top to button along a group the
size of the atom and the shielding effect of the inner orbital increases due to
addition of new shell and sub shell.
Again the nuclear charge of the atom also
increases. But the combined effect of size increase
and shielding effect of is more effective than the effect of increasing the
nuclear charge.
Hence, for any group
when we go down the group the ionization
energy gradually decreases. That
is, ionization energy of elements
decreases from top to button along a group.
Factors affecting ionization energy
Ionization energy of element depends on some factor of atom, such as atomic size, the extent of nuclear charge, shielding effect of inner orbital, penetrating power of orbital, half filled or fulfilled orbital and electronic configuration of outer most shell etc.
Atomic size:
Ionization energy is inversely proportional to the size of atom. If the size of the atom is small, then the force of attraction of nucleus towards outer most electron increases. Hence ionization energy increases.
Nuclear charge of atom:
If the other causes remain unchanged, then ionization energy is directly
proportional to the nuclear charge of the atom.
With increase in nuclear charge of atom the force attraction between nucleus and outer most electron increases and hence ionization energy increases.
Screening effect of inner atomic orbital:
Inner orbital with completely electron filled act as a screen between nucleus and outer electronic shell of atom.
As a result, the force of attraction of nucleus to
outer most shell is less. That is, effective nuclear charge of nucleus is less
than its real value of charge.
Consequently, the value of ionization energy is less than expected value. The power or tendency of inner electron-filled shell shield the nuclear charge is called the screening effect. The order of shielding power of different orbital are s> p>d>f.
Penetrating
power of orbital: It has been found that different orbital
shows different penetrating power due to their different shape.
For example, s-orbital is more penetrating towards
nucleus than p-orbital, p-orbital is more penetrating towards nucleus than
d-orbital and so on.
Hence s-orbital or s-electron is more attracted by nuclear charge and f-orbital is least attracted by nucleus.
Due to the above said reason, the order of ionization energy of different sub shell of a particular shell is s>p>d>f.
Effect of half filled or full filled orbital:
According
to Aufbau principle, half filled or full filled orbital is more stable than
less than or more than half filled or full filled orbital.
Therefore,
the amount of energy to remove an electron from half filled or full filled
orbital must be higher than the energy required to remove an electron from less
than or more than half filled or full filled orbital.
That is, the ionization energy of atom with half filled or full filled orbital is higher than the ionization energy of atom with less than or more than half filled or full filled orbital.
Effect of
electronic configuration of outer most shell:
The
atom with electronic configuration of outer most shell ns2np6 is more stable
than the others electronic configuration. That is, atom with complete octet is
more stable and inert.
So
the energy required to remove an electron from such type of atom is much higher
than the other atom. Hence the value of ionization
energy for such type of atom is greater higher than the other atoms.
Significance or importance or application of ionization energy
Many
important properties of the element are known from the value of the ionization
potential. For example, the
metallic and non-metallic properties of elements can be known from the values
of ionization energy.
If the value of ionization energy of atom is low, then
the element is metal and if it is high, it will be non-metal.
Again, the number of valence
electrons present in the atom of an element can be determined from the value of
ionization potential.
For
example, the value of first and second ionization
energy of Li are 5.4 eV and
75.6 eV respectively. From this value it is clear that the first electron of Li
is easily released. So it is valence electron.
The chemical activity of the
elements can be explained with the help of their ionization potential value. When the ionization potential of an element is low, the element
becomes a very active metal.
But if the value of ionization potential is
too high, the element becomes inactive. Though, this rule is applicable only
for metallic atoms.
The value of ionization energy can be used to
explain the reducing power of an element. If the value of the ionization
potential of the element is low, then the element shows reduction properties.
The alkaline properties of
the elements are known from the value of their ionization energy. When the ionization potential of an element is
low, the element is a very active metal and of an alkaline nature.
The value of the ionic
potential of the elements determines whether the reaction of the two elements
will produce an electrovalent or covalent compound.
If the difference in the
value of the ionic potential of the two elements is too high, then an
electrovalent compound is formed. But if the difference in ionic potential values of the two elements
is not too high, then covalent compounds are formed.
Why is the ionization
energy of Na+
higher than Ne?
In the case of Na +
ions, the number of protons is 11 and the number of electrons is 10. On the other hand, in the case of the Ne atom, the number of
protons is 10 and the number of electrons is also10.
It is clear that the
effective nuclear charge of Na+ ion is higher than that of Ne element. Thus in the case of Na + ions, the force of attraction of the
nucleus towards the outer electron acts more.
Consequently, the amount
energy required to remove one more electron from Na+ is higher than that of Ne
atom. That is, the ionization
energy
of Na+ is higher than Ne atom.
Why is the ionization energy of nitrogen higher than oxygen?
In
general, ionization energy of
elements is directly proportional to the electronegativity of the elements
(excluding inert gas). Now oxygen is more electronegative than nitrogen.
Hence,
the ionization energy of oxygen
should be higher than nitrogen. But the actual order is reversed. That is, the
ionization potential of nitrogen is higher than oxygen.
This
anomalous can be explained on the basis of their electronic configuration.
Nitrogen is a p3 system whereas oxygen is p4 system.
According
to Aufbau principle, half filled or full filled orbital is more stable than
less than or more than half filled or full filled orbital.
Since
nitrogen atom occupied exactly half filled p-electron hence nitrogen is more
stable than oxygen.
So, the amount of energy required to remove an electron from 2p-orbital of nitrogen atom is greater than that of oxygen atom. Consequently, the ionization energy of nitrogen becomes higher than oxygen.
Why is the ionization energy of phosphorous higher than sulfur?
From
the periodic variation of ionization
energy, it is known to me that with increase in nuclear charge ionization energy of elements increases.
Hence, the ionization energy of sulfur (16) atom should be higher than phosphorous (15) atom. But the actual order is reversed. That is, the ionization potential of phosphorous atom is higher than sulfur atom.
This
anomalous can be explained on the basis of their electronic configuration.
Phosphorous is a p3 system whereas sulfur is p4 system.
According
to Aufbau principle, half filled or full filled orbital is exceptionally stable
than less than or more than half filled or full filled orbital.
Since
phosphorous atom occupied exactly half filled 3p-electron hence phosphorous is
more stable than sulfur atom.
So,
the amount of energy required to remove an electron from 3p-orbital of
phosphorous atom is higher than that of sulfur atom.
Consequently,
the ionization energy of phosphorous
atom becomes higher than sulfur atom.
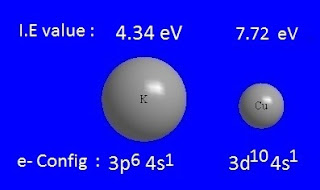
Why is the I.P of Cu higher than that of K, even though both have 4s1 electrons in their outer orbital?
The outermost shell of
the K-atom does not have an inner d-sub shell. But the Cu-atom has a 3d-sub shell.
Now, due to the less
shielding effect of the d-sub shell, the 4s electron of the Cu-atom is more
strongly attracted by the nucleus.
As a result, more energy is
needed to isolate the 4s electron.
For
this reason, the ionization energy
of Cu is higher than that
of K, even though both have 4s1 electrons in their outer orbital.
- Why is the ionization potential of Na+ higher than Ne?
- Why is the ionization potential of Na+ higher than Na?
- Why is the ionization potential of nitrogen higher than oxygen?
- Why is the ionization potential of phosphorous higher than sulfur?
- Why is the I.P of Cu higher than that of K, even though both have 4s1 electrons in their outer orbital?
Ionization energy, ionization
enthalpy, ionization potential, periodic variation of ionization energy,
factors affecting ionization energy, IE of Cu and K, IE of P and S, IE of Na+
and Ne.











No comments:
Post a Comment