What is the molecular weight of methanol?
Methanol,
also known as methyl alcohol, is an organic compound with molecular formula
CH4O or CH3-OH.
It
is simplest aliphatic primary alcohol. Methanol is a colorless, inflammable and
poisonous liquid.
However,
to determine the molecular weight of methanol, first we have to know the
molecular formula of the methanol molecule.
Then the number of different atoms in a molecule of methanol has to be determined. Besides, we need to know the atomic mass of different atoms.
This
is because the summation of atomic mass of atoms present in a methanol molecule
is the molecular weight of methanol.
Read more : What is methanol or methyl alcohol?
Read also : What is urea fertilizer in chemistry?
What is molecular weight or molecular mass?
The
summation of atomic mass of atoms present in a molecule
of a compound is the molecular weight or molecular mass of that compound.
There
are three scale for determination of molecular weight or molecular mass, namely
carbon scale, hydrogen scale and oxygen scale.
According to carbon scale, the mass of a molecule
of a compound is as much as the mass of an atom of carbon C-12, that number is
called the molecular mass of that compound.
Structure of methanol
Molecular
weight determination and many others analysis show that the molecular formula
of methanol is CH4O.
That
is, methanol contains one carbon atom, four hydrogen atoms and one oxygen atom.
Assuming
that carbon atom is tetra valent, oxygen bivalent and hydrogen univalent, only
one structure is possible which is shown.
The
above chemical formula as well as structure of methanol is supported by all the
chemical reactions of methanol.
For
example, only one hydrogen atom in methanol is replaced by sodium. This
suggests that one hydrogen atom is in a different state of combination from the
other three.
Methanol
is formed from methyl chloride by hydrolysis with NaOH. Methyl chloride can
have only the structure CH3-Cl.
This
means that the methyl group in methyl chloride is unchanged by the action of
dilute alkali, and that the reaction takes place by the replacement of the ‘Cl’
atom by a –OH group.
Now,
the presence of –OH group is confirmed by the reaction between methanol and
PCl5, when CH3-Cl, HCl and POCl3 are formed.
Thus
one oxygen atom (bivalent) one hydrogen atom (univalent) have been replaced by
one chlorine atom (univalent).
This
implies that the oxygen and hydrogen atoms exist as a univalent group in
methanol.
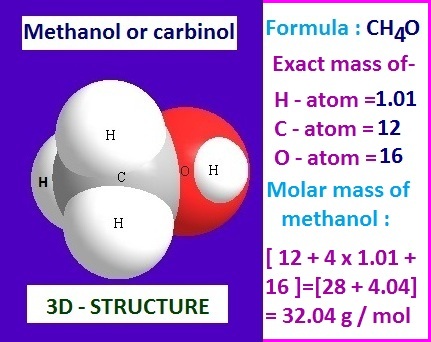
Methanol molecular weight
From
the above experimentally discussion, it is clear that methanol contain one
carbon atom, four hydrogen atoms and one oxygen atom in its single molecule.
According
to definition, the molecular weight of methanol is the summation of atomic
weight of carbon, hydrogen and oxygen atoms.
Now
the atomic weight of carbon atom, hydrogen atoms and oxygen atom from their
mass diffraction literature value are 12, 1.01 and 16 respectively.
Therefore,
the molecular weight of methanol should be [12 + 4 x 1.01 + 16].
That
is, [28 + 4.04] = 32.04.
Hence
from the above calculation, it has been found that the exact molecular weight
of methanol is 32.04. That is 32.04 g/mol.
- What is the molecular weight of methanol?
- What is methanol molecular weight?
- What is methanol molar mass?
- What is the molar mass of methanol?
methanol
molecular weight, molecular weight of methanol, methanol molar mass, molar mass
of methanol,
Read more : What is urea fertilizer in agriculture?










No comments:
Post a Comment