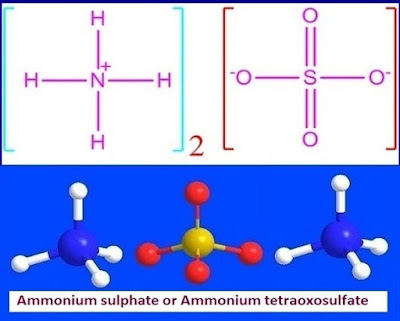
What is ammonium sulfate or (NH4)2SO4?
Ammonium
sulfate is an inorganic compound with chemical formula, (NH4)2SO4. It is
nitrogen as well as sulfur containing manure and is predominantly used in
agriculture.
Ammonium
sulfate is also known as ammonium tetraoxosulfate, diammonium sulfate, mascagnite,
dolamin, actamaster, sulfuric acid diammonium sulfate etc.
It
is fine white hygroscopic granules or crystals and soluble in water but
insoluble in acetone, ether or alcohol.
The
solubility of ammonium sulfate in water increases with increase in temperature.
The density of ammonium sulfate is 1.76 g/cc and it melts at 508 to 533K.
Ammonium sulfate preparation
Ammonium
sulfate is prepared by treating NH3 with concentrated sulfuric acid. This
reaction is carried out at a 333K fixed temperature.
Concentrated sulfuric acid is added to keep the solution acidic, and to retain its level of free acid.
2
NH3 + H2SO4 → (NH4)2SO4
Dry,
powdered ammonium sulfate may be formed by spraying sulfuric acid into a
reaction chamber filled with ammonia gas.
Ammonium
sulfate also is manufactured from gypsum. When finely divided gypsum is added
to an ammonium carbonate solution, then ammonium sulfate along with CaCO3 is
obtained.
(NH4)2CO3
+ CaSO4 → (NH4)2SO4 + CaCO3
What is ammonium sulfate fertilizer?
Ammonium
sulfate is a nitrogen and sulfur containing inorganic salt. It contains 21.2%
nitrogen and 24.2% sulfur elements. It
provides the nitrogen needed for plant growth.
Ammonium
sulfate plays an important role to increase the acidity of alkaline soils by
releasing ammonium ions and maintains the pH balance in the soil.
So
ammonium nitrate is very important fertilizer and is predominantly used in
agriculture for plants.
Ammonium sulfate pH
Ammonium
sulfate is neither acid nor a base. It is a salt of ammonia and sulfuric acid.
So on hydrolysis, ammonium sulfate produces ammonium hydroxide and H2SO4.
Now,
sulfuric acid is a strong acid but NH4OH is a weak base. Hence, the aqueous
solution of ammonium sulfate is overall acidic.
Consequently,
the pH of (NH4)2SO4 is less than 7. It has been found that, the pH of 0.1 M ammonium
sulfate solution is 5.5.
Ammonium sulfate uses
Ammonium sulfate uses in agriculture
Ammonium
sulfate is a nitrogen and sulfur containing fertilizer. It contains 21.2%
nitrogen and 24.2% sulfur elements.
So
ammonium nitrate is very important fertilizer for plants growth. For this
reason, it is predominantly used in agriculture for plants.
Generally,
ammonium sulfate is used as fertilizer in alkaline soils. This is because it
releases ammonium ions into the soil and forms small amounts of acid.
This
maintains the pH balance in the soil. It
also provides the nitrogen needed for plant growth.
It
is also used as an agricultural spray adjuvant for water-soluble insecticides,
herbicides, and fungicides.
There,
it functions to bind iron and calcium cations that are present in both well
water and plant cells.
It
is particularly effective as an adjuvant for 2, 4-D (amine), glyphosate, and
glufosinate herbicides.
Laboratory use
Ammonium
sulfate precipitation is a common method for protein purification by
precipitation. It is used as an important laboratory reagent.
Ammonium
sulfate is used in group analysis for group metals. In the analysis of rubber
lattices, volatile fatty acids are analyzed by precipitating rubber with a 35%
ammonium sulfate solution.
Ammonium sulfate use as food additive
Ammonium
sulfate is used a food additive. Again, it is used as an acidity regulator in
flours and breads.
Other uses
In the treatment of drinking water, ammonium sulfate is
used in combination with chlorine to generate monochloramine for disinfection.
A small scale of ammonium sulfate is used in the preparation
of other ammonium salts, especially ammonium persulfate. Ammonium sulfate is
also used as a wood preservative.
- What is ammonium sulfate or (NH4)2SO4?
- Ammonium sulfate preparation
- What is ammonium sulfate fertilizer?
- Ammonium sulfate pH
- Ammonium sulfate uses
- Ammonium sulfate uses in agriculture
ammonium
sulfate, ammonium sulfate fertilizer, ammonium sulfate pH, ammonium sulfate
uses, ammonium sulfate uses in agriculture,
Read more: What is acid-base neutralization?










No comments:
Post a Comment