What is the shape and magnetic properties of Fe(CO)5?
The
atomic number of iron is 26. The oxidation of state of Fe in Fe(CO)5 compound
is zero. Now, the ligand CO is a very strong field ligand.
Hence
due to influence of strong field CO ligand the four unpaired electron of 3d sub
shell of Fe(0) becomes paired and two 4s electron is shifted to 3d sub shell.
As
a result, 3d sub shell of Fe(0) occupied four paired electron in its four
orbitals and the rest one orbital remain vacant.
This
vacant 3d-orbital is mixed with one 4s and three 4p orbitals, resulting in the
formation of five energetically equivalent dsp3
hybridized orbitals.
These
vacant five hybridized orbitals accept five pair of electrons from five strong
field CO ligand forming five co-ordinate bond, resulting in the formation
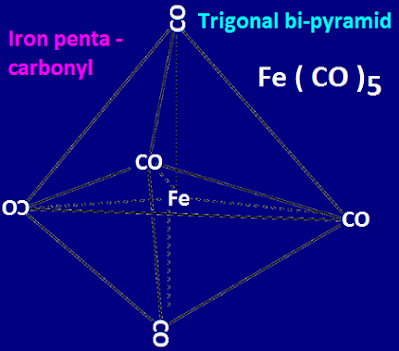
tri-gonal bi-pyramidal Fe(CO)5 molecule.
Since
there is no unpaired electron in 3d-orbital of central metal Fe-atom, hence the
resulting Fe(CO)5 compound shows diamagnetic properties.
However,
the electronic configuration according to Hund’s rule the hybridization and
shape of iron penta carbonyl compound is shown below.
- What is the shape and magnetic properties of Fe(CO)5?
- What is the geometry and magnetic properties of Fe(CO)5?
- Why Fe(CO)5 has tri-gonal bi-pyramid structure?
- What is the hybridization of Fe(CO)5?
- Why is Fe(CO)5 diamagnetic in nature?
Shape
and magnetic properties of Fe(CO)5, geometry and magnetic properties of Fe(CO)5,
hybridization of Fe(CO)5, Fe(CO)5 has tri-gonal bi-pyramid structure, Fe(CO)5
diamagnetic in nature,
Read more : Main causes of global warming










No comments:
Post a Comment