Why is [ NiCl4 ] 2– paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic ?
The
Ni(II) ion is 3d8 system.
According to Hund’s rule the outer
electronic configuration of Ni(II) ion
is [ Ar ] 3d8. From
electronic configuration of Ni(II) ion , it is shown that it has two unpaired electrons.
Now,
depending upon the hybridization, there are two types of possible structure of Ni(II) complex are formed with co-ordination number 4.
If
the complex involves ‘sp3’ hybridization, it would have
tetrahedral structure . Again, If the complex involves ‘dsp2’ hybridization, it would have square planar structure .
Consequently,
for the formation of tetrahedral structure through the ‘sp3’ hybridization, the
3d-orbital of nickel atom remain unaffected.
Therefore,
3d-orbital of nickel(II) ion possessed two unpaired electrons and hence the
concern complex would be paramagnetic.
On
the other hand, for the formation of square planar structure through the dsp2
hybridization, one of the 3d-orbital of nickel atom should be empty and
available for hybridization.
But,
it is possible if the two unpaired 3d-electrons are paired up due to the energy
made available by the approach of ligands.
Under
this condition, all the 3d-electrons of Ni(II) ion are paired and hence the
complex would be diamagnetic.
Now,
in case of [ NiCl4 ] 2–complex ion, Ni(II) ion with co-ordination 4 involves ‘sp3’ hybridization. Hence the geometry of, [ NiCl4 ] 2–complex ion would be tetrahedral.
Under
this condition, the electronic arrangement of
Ni(II) ion is evidently shown that the 3d-orbitals of Ni(II) ion have '2' unpaired electrons and hence[ NiCl4 ]2–complex ion is paramagnetic.
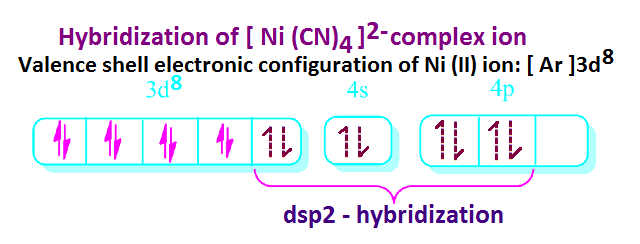
On
the other hand, in case of [ Ni ( CN ) 4
] 2– complex ion, Ni(II) ion with co-ordination number 4 involves ‘dsp2’ hybridization.
Hence
the geometry of, [ Ni ( CN ) 4
] 2– complex ion would be
square planar.
The
empty hybrid orbitals of metal overlap with fully filled orbitals of cyanide
ion to form metal-ligand co-ordinate bond.
Under
this condition, the electronic arrangement of Ni(II) ion is evidently shown
that there is no unpaired electrons
present in 3d-orbitals of Ni(II) ion
and hence [Ni ( CN ) 4 ] 2–
complex ion is diamagnetic.
- Why is [ NiCl4 ] 2– ion paramagnetic while [ Ni(CN) 4 ] 2– ion is diamagnetic ?
- Why is [ NiCl4 ] 2– ion paramagnetic in nature?
- Why is [ Ni(CN) 4 ] 2–ion paramagnetic in nature?
- Why is [Fe(CN)6]3–a low spin complex ?
- Why is [Fe(H2O)6]3+a high spin complex ?

![[ NiCl4] 2– is paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic [ NiCl4] 2– is paramagnetic while [ Ni(CN) 4 ] 2– is diamagnetic](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhwbwYzbTnE9yhRuMJRUzMb5kJIDBF1EuZr6NMga3PUHTOg5eoFtr7Av-1ybb41axuaGCmpmQRfsWt3xlDHkdVPBcNCNdgMXZbuF7jinN7ioyLqXebiN_at60fQr_G4enCvRYumnlKubek/w640-h284/bSE.jpg)









No comments:
Post a Comment