What is N-bromo succinimide or NBS ?
N-bromo
succinimide or NBS is a very
important chemical reagent which is used in radical substitution ,
electrophilic addition and electrophilic substitution reactions in organic chemistry . It is used mainly as a
allylic and benzylic bromination . The
structure of N-bromo succinimide or NBS
is as follows,
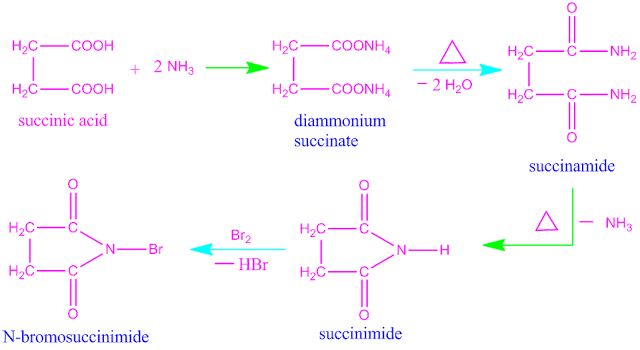
Preparation of N-bromo succinimide or NBS .
N-bromo
succinimide is prepared from succinic acid. When succinic acid is reacts with
ammonia it forms diammonium succinate which on heating gives succinamide . On
further heating succinamide convert into succinimide .This succinimide reacts
with bromine gives N-bromo succinimide or NBS .
What is the use of N-bromo succinimide or NBS ?
N-bromo
succinimide or NBS is a very
important chemical reagent , which is used in the preparation of
aliphatic and aromatic halo alkane and halo arenes .
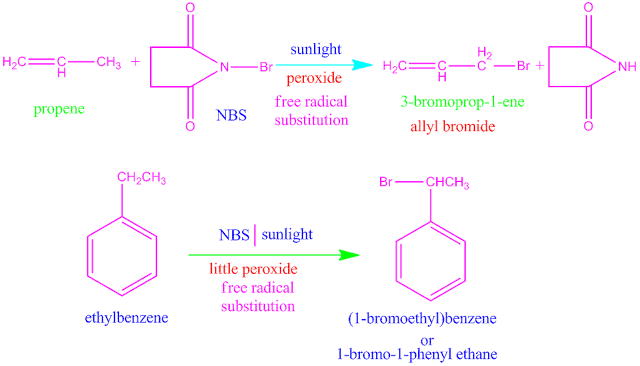
N-bromo
succinimide or NBS is a good reagent for
allylic and benzylic bromination . For example,
when NBS reacts with propene in the presence of sunlight and peroxide ,
3-bromoprop-1-ene is the product which obtained in large extent .
Again , when
NBS reacts with ethyl benzene in the presence of sunlight and little peroxide, 1-bromo-1-phenyl ethane is
obtained .
In both
cases the reaction proceed through free radical substitution method .
Besides the
above reaction NBS is used in bromination of carbonyl derivatives and
also in Hofmann rearrangement reaction .
Why SOCl2 is more suitable than PCl5 to prepare chloro alkane from alcohol ?
To prepare
chloro alkane from SOCl2 is more suitable reagent than PCl5
, because if we use SOCl2 , the side products are SO2 and
HCl . Both they are in gaseous state. So they are easily eliminated from the
product mixtures . As a result , we can get about pure chloro alkane.
On the other
hand if we used PCl5 instead of SOCl2 ,the side products
are POCl3 and HCl . Although HCl is in gaseous form , but POCl3
can not be separated from the products mixture .
Consequently,
pure chloro alkane is not available.
Summary :
What is N-bromo succinimide or NBS ?
Preparation
of N-bromo succinimide or NBS .
What is the
use of N-bromo succinimide or NBS ?
Why SOCl2 is more suitable than PCl5
to prepare chloro alkane from alcohol ?










No comments:
Post a Comment