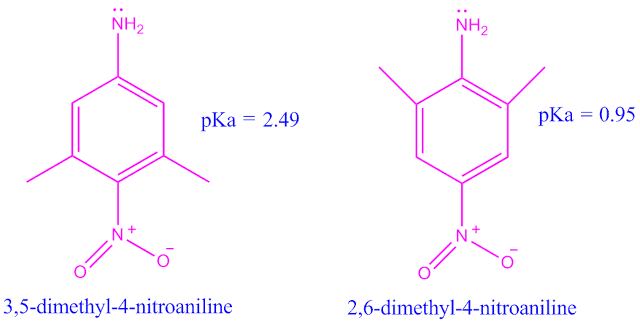
Why 3,5-dimethyl 4-nitro aniline is stronger base than 2,6-dimethyl 4-nitro aniline ?
3,5-dimethyl 4-nitro aniline is a stronger base than 2,6-dimethyl 4-nitro aniline because, in 3,5-dimethyl 4-nitro aniline, the nitro group can not enter into resonance due to steric inhibition of resonance .
But in 2,6-dimethyl 4-nitro aniline, the nitro group can take part into resonance with amino group.
Although a steric effect is operating between the amino group and the two ortho-methyl groups, yet in this case, since only hydrogen atoms are involved, the steric effect will be very much smaller than that due to the oxygen atom.
Why o-cresol is more acidic than 2,6-dimethyl phenol ?
The strength of acid depends on the stability of conjugate base of that acid.
The 2,6-dimethyl phenoxide ion is less stable than o-methyl phenoxide ion due to additional +I effect of two methyl groups in 2,6-dimethyl phenol . Hence, o-cresol is more acidic than 2,6-dimethyl phenol .
Why 3,5-dimethyl 4-nitro phenol is more acidic than 3,5-dimethyl phenol ?
In 3,5-dimethyl 4-nitro phenol, the nitro group is strong electron withdrawing group.
So the lone pair on oxygen atom takes part in resonance with the nitro group through pi electron of benzene nucleus.
Hence, O―H bond becomes more ionic than that of 3,5-dimethyl phenol.
Consequently, 3,5-dimethyl 4-nitro phenol can release proton easily.
For the above reason,3,5-dimethyl 4-nitro phenol is more acidic than 3,5-dimethyl phenol.
Practice problem
- Why phenol is more acidic than aliphatic alcohol ?
- Why p-nitro phenol is stronger acid than phenol ?
- Why picric acid is more stronger acid than phenol ?











No comments:
Post a Comment