What is E1cB-reaction mechanism?
The E1cB-reaction mechanism stands for elimination uni molecular conjugate base.
The E1cB-elimination reaction is a type of 1,2-elimination reaction which occurs under basic conditions, where a poor leaving group and an acidic β-hydrogen are eliminated from a substrate leading to formation of an unsaturated compounds or alkene.
The E1cB-elimination reaction is a type of 1,2-elimination reaction which occurs under basic conditions, where a poor leaving group and an acidic β-hydrogen are eliminated from a substrate leading to formation of an unsaturated compounds or alkene.
This is a two step base catalysed 1,2-elimination reaction.
In first step, the most acidic β-H atom of concerned substrate is eliminated as a proton by the attact of base resulting in the-
formation of a stable carbanion ,which is also a conjugate base of substrate.
formation of a stable carbanion ,which is also a conjugate base of substrate.
In second step, the leaving group from α-carbon is eliminated as a anion leading to formation of an unsaturated compounds or alkene.
The second step involves a slow unimolecular elimination of conjugate base of substrate, hence the mechanism is called E1cB.
It has been experimentally proved by applying kinetic isotope effect and steady-state concept that, the The E1cB mechanism follows second order kinetics.
Thus, the rate of reaction =k[
substrate] [conjugate base].
Example of E1cB reaction.
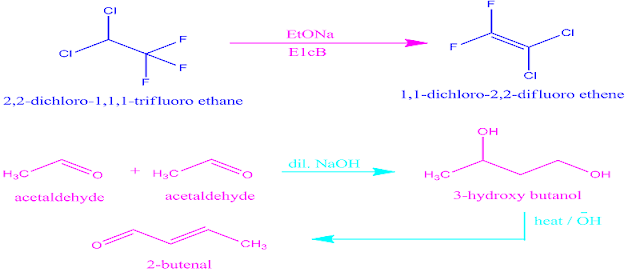
Reaction of 2,2 dichloro-1,1,1-trifluoro ethane with sodium ethoxide is the most important example of E1cB reaction .
E1cB reaction mechanism.
The another most well known reaction that
undergoes E1cB elimination is the aldol condensation reaction under basic conditions.
This involves the deprotonation of a compound containing a carbonyl group that results in the formation of a carbanion.
The carbanion is the very stable conjugate base of substrate, and is one of the intermediates in the reaction.
This carbanion then acts as a nucleophile and can attack an electrophilic aldehyde.
The Aldol product is then deprotonated forming another carbanion followed by the elimination of water in an E1cB dehydration reaction and form an alkene.
This involves the deprotonation of a compound containing a carbonyl group that results in the formation of a carbanion.
The carbanion is the very stable conjugate base of substrate, and is one of the intermediates in the reaction.
This carbanion then acts as a nucleophile and can attack an electrophilic aldehyde.
The Aldol product is then deprotonated forming another carbanion followed by the elimination of water in an E1cB dehydration reaction and form an alkene.
Example of E1cB reaction.
E1cB reaction mechanism.
The energy profile diagram of E1cB mechanism is shown below.
(II)What is alpha(α)-elimination : There are few example of elimination reaction, where two atom or groups are eliminated from a single carbon atom of substrate molecule, such type of elimination reaction is called α-elimination reaction.
Example:
(III)What is gama(𝝲)-elimination : There are few example of elimination reaction, where two atom or groups are eliminated from α and Ƴ position of carbon atom of substrate molecule, such type of elimination reaction is called Ƴ–elimination reaction.
Example:
Summary :
What is E1cB-reaction mechanism ?
What is alpha-elimination ?
What is gama-elimination ?















No comments:
Post a Comment