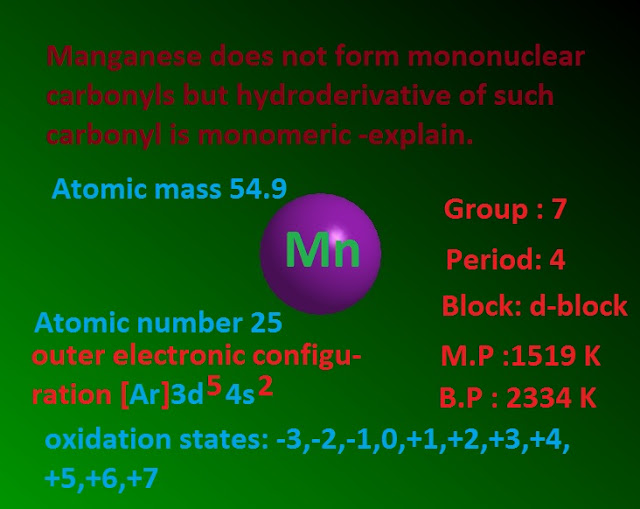
Why manganese does not form mono nuclear carbonyl compound but hydro derivative of such carbonyl is monomeric – explain ?
The bonding in metal carbonyls can be explained with the help of valence bond theory . But the strength of metal-carbonyl bond in carbonyl compounds can only be explained with the help of molecular orbital theory
According to valence bond theory every element has the
tendency to attain the next inert gas electronic configuration during compound
formation .In carbonyls, also the transition metals are involved to attained
the next inert electronic configuration.
The atomic number of the next inert gas is termed as effective atomic number ( or EAN ). In all the metal carbonyl compounds , EAN rules is followed. Thus, the type of the metal carbonyl either mono nuclear or poly nuclear , can be easily determined with the help of EAN rule.
The numbers 36(Kr), 54(Xe), and 86 (Rn ) are the EAN of 3d, 4d and 5d series transition metal having even atomic number can be easily form a mono nuclear carbonyl compound .
The atomic number of the next inert gas is termed as effective atomic number ( or EAN ). In all the metal carbonyl compounds , EAN rules is followed. Thus, the type of the metal carbonyl either mono nuclear or poly nuclear , can be easily determined with the help of EAN rule.
The numbers 36(Kr), 54(Xe), and 86 (Rn ) are the EAN of 3d, 4d and 5d series transition metal having even atomic number can be easily form a mono nuclear carbonyl compound .
Since CO is a two
electron donar so EAN
is attained easily. That is why Ni ( CO )4
(28+2x4=36) , Fe ( CO )5 (26+ 2x5=36 ) and Cr(CO )6 (28+2x4=36 ) are known.
But Mn and Co can’t form a mono nuclear carbonyls compounds
because they have odd atomic number and it is not possible to attain EAN rules.
If one assumes that Mn ( CO )5 can be prepared then it would behave as a
free radical [ 25+2x5 =35 , having one unpaired electron ] and will
dimerise easily .
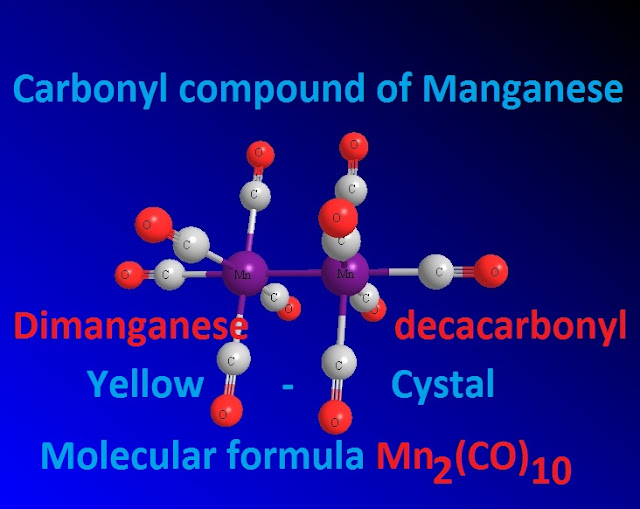
That is why manganese does not form mono nuclear carbonyl compound , but it forms the bimolecular carbonyl compounds like Mn2 (CO )10 which when reduced with hydrogen the hydro derivative HMn( CO )5 is produced, it is very stable because it obeys EAN rules .
That is why manganese does not form mono nuclear carbonyl compound , but it forms the bimolecular carbonyl compounds like Mn2 (CO )10 which when reduced with hydrogen the hydro derivative HMn( CO )5 is produced, it is very stable because it obeys EAN rules .
Practice problem:
Why manganese shows various oxidation states ?
What is effective atomic number (EAN) rules ?









No comments:
Post a Comment