What is methane gas in organic chemistry?
The first compound in the paraffin or alkene class is methane gas. Methane gas is also known as sweet gas or Marsh gas.
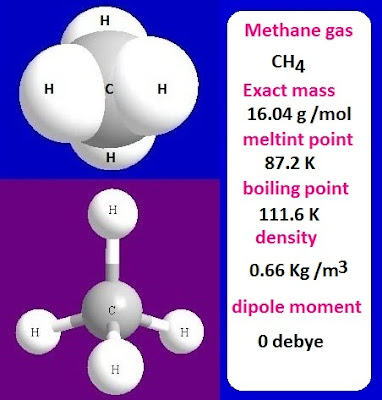
Its molecular formula is CH4.
Methane gas is the main component of the
natural gas that is obtained from underground oil wells. Methane gas is 90% of natural gas.
Methane gas is also found in coal mines. Coal gas produced as a result of internal
distillation of wood also contains about 50% methane gas.
Methane gas is not toxic. It is a colorless, odorless,
tasteless gas. Methane
gas is much lighter than air. Its vapor density is about half that of air vapor
density.
Methane
is a non polar gas and hence insoluble in water but soluble in organic
solvents, such as, alcohol, ether, benzene, acetone, toluene etc.
When cooled under pressure at critical temperatures, methane gas first
becomes liquid and then solid. The melting point of methane gas is 87.2K and the
boiling point is 111.6K.
The
density of methane gas is 0.66 kg / cubic meter at 25ᵒC temperature and 1
atmosphere pressure.
Read also : What is methanol or methyl alcohol?
Chemical formula and structure
Mass
diffraction and other analysis proved that the chemical formula of methane gas
is CH4. In methane, the central carbon atom is directly attached with four hydrogen
atoms.
The
carbon atom in methane is sp3 hybridized. The geometry of the methane molecule
is tetrahedral. The H – C – H bond angle is 109ᵒ28’ and C – H bond distance is
1.09Å.
All
the four H-atoms in methane are equivalent. Because of substitution of one H-
atom by any univalent atom or group give only one mono substituted compound.
This implies that all four H-atoms of methane are equivalent.
Methane gas preparation
There
are different methods for the preparation of methane gas. Out of them, laboratory method and synthetic
method are most important.
Laboratory method
In
laboratory method, anhydrous sodium acetate is mixed with dry sodalime by 1:3
rations and then heated. As a result, methane gas is obtained.
Synthetic
method
In
this method, a mixture of CO or CO2 and hydrogen is passed over a Ni dust at
573K – 673K temperature and as result of this methane gas is obtained.
Others
method (room temperature method)
At
room temperature, on reduction of CH3-I by ethyl alcohol and Zn-Cu couple pure
methane is obtained. Again, methane gas is also prepared by hydrolysis of
Al4C3.
What is marsh gas?
The
word Mars means rotten swamp. Methane gas is called Marsh gas. This is because
methane gas is produced in the decomposition of plants and animals in ponds,
beels and wetlands.
That is, methane gas is produced by the bacterial decomposition of cellulose
obtained from plants.
What is will-o-the wisp or Aleya?
Many
times methane gas is produced as a result of bacterial decomposition of animals
with plants in decomposing muddy wetlands.
It
also produces small amounts of phosphine and phosphorus dihydride. Phosphorus dihydride is a very flammable substance.
It ignites when it comes in contact with air.
As a result, both methane and phosphine are
combustible and ignite and produce light. This
is called will-o-the wisp or Aleya.
Uses of methane gas
Methane
gas is the main component of compressed natural gas (CNG), which is used as an
excellent fuel in different sector for different purpose.
As
a fuel, methane gas is excellent. This is because methane gas causes less air
pollution than other fuels. Again, it has high calorific value.
For
this reason, methane gas is used as fuel in ovens, water heaters, furnaces,
cars, turbines and other things. It is also used to keep the home warm.
As a major component of CNG, methane gas is important for
power generation by burning as fuel in turbines or steam generators. In many cities,
methane is piped to households for domestic heating and cooking.
As
rocket fuel, methane gives advantage over the production of small exhaust
molecules over kerosene.
There are many other uses of methane gas in organic
chemistry. For example, CO2 obtained from methane's heat dissipation is used to
make paint, printing inks and to make car tires.
Many
necessary chemicals such as methyl chloride, acetylene, formaldehyde and
methanol are produced from methane gas.
The synthesis gas (CO + H2) obtained by the reaction of steam with
methane is used in the production of methanol and hydrogen gas.
- What is methane gas in organic chemistry?
- What is marsh gas?
- What is will-o-the wisp or Aleya?
- Uses of methane gas
methane gas, marsh gas, sweet gas,
will-o-the
wisp or Aleya, use of methane gas, methane gas formula and structure,










No comments:
Post a Comment