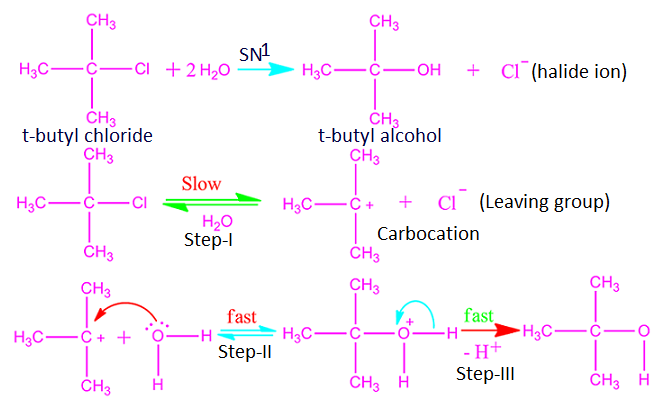
Why do polar solvent and weak nucleophiles favor SN1 reaction?
Polar
protic solvents, such as CH3OH, CH3CH2-OH, H2O, etc., are suitable for SN1
reactions. This is because in this type of solvent, both carbocation and halide
anion gain stability. That is, polar
protic solvent favor SN1 reaction mechanism.
The strength of nucleophile depends on the electronegativity of the concern attacking atom. As the electronegativity of the atoms of the element increases, the tendency to donate electrons to others decreases.
As a result nucleophilicity decreases. That is, it acts as a weak nucleophile. When we move from left to right along a period in periodic table, the electronegativity of the atoms increases and hence nucleophilicity of nucleophile decreases.
For example, among CH3-, NH2-, OH- and F-, CH3- act
as a strong nucleophile and F- act as a weak nucleophile. Now, it is observed
that weak nucleophile favor SN1 reaction
mechanism.
This is because the attacking atom of weak
nucleophile becomes more electronegative as well as more electron rich.
On
the other hand the carbocation has a center with more positive charge. More electropositive charge centers are
attacked by more electronegative charge centers. Consequently, do polar solvent and weak nucleophile favors SN1 reaction.
- Why do weak nucleophiles favor SN1 reaction mechanism?
- Why do polar solvent favor SN1 reaction mechanism?









No comments:
Post a Comment