What is oxidation of iron in inorganic chemistry?
The
standard reduction potential of iron is -0.44 volt which is less than alkali
metals and alkaline earth metals.
Therefore,
oxidation of iron metal is not so easy with respect to the above said metals.
But oxidation of iron takes place under certain conditions.
Oxidation
of iron may occur naturally as well as by artificially. However, oxidation of
iron takes place in the presence of air or oxygen and forms ferric oxide,
ferrosoferric oxide, ferrate etc compounds.
There is no reaction of iron with dry air. But iron in humid
air is oxidized by oxygen of air.
This results in a brown coating on the iron, called rust. Rust is hydrated ferric oxide. Its symbol is Fe2O3.3H2O.
The oxidation number of iron in this compound is +2. That is, the iron
has lost two electrons. So the iron has been oxidized. This is natural
oxidation of iron.
Oxidation of iron reaction equation
If iron is heated intensely in the presence of air or oxygen it ignites by producing sparks.
In
this case also the oxidation of iron occurs. Iron is oxidized by oxygen to
produce ferrosoferric oxide [FeO.Fe2O3], in which the mean oxidation number of
iron metal atom +8/3.
Hence
in this case oxidation of iron has been taken place. The oxidation reaction is
shown below.
Under normal conditions iron does not react with alkali. But iron reacts with strong alkalis like NaOH, KOH
etc. to produce ferrate compounds.
For examples, sodium ferrate, potassium
ferrate etc. The oxidation number of iron in ferrate compounds is +3.
That
is, the iron atom has lost three electrons. Thus
iron is oxidized indirectly by oxygen.
What is oxidation of iron in water?
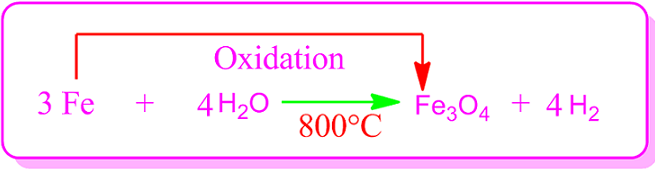
The
most important oxidation reaction of iron is the reaction with steam. Under normal conditions, iron does not react with
water.
But when steam is run over red hot iron at a temperature of about 800ᵒC, ferrosoferric oxide and hydrogen are
produced.
In this case iron is oxidized
and converted into ferrosoferric oxide [FeO.Fe2O3], in which the mean oxidation
number of iron metal atom +8/3.
Hence
in this case oxidation of iron has been taken place. The oxidation reaction is
shown below.
- What is oxidation of iron in inorganic chemistry?
- What is oxidation of iron in water?
- Oxidation of iron in brown ring complex test
- Oxidation of iron reaction equation
Oxidation of iron, oxidation of iron in water,
oxidation of iron reaction equation, oxidation of iron equation, oxidation of
iron causes, oxidation of iron brown ring complex test, oxidation of iron
formula,












No comments:
Post a Comment