What is thermodynamically controlled product?
When a chemical
reaction gives rise to alternative
products ,the nature of the products and their proportion in the
products mixture depends on the energetic of the reaction, that is, on the kinetics and the thermodynamic of the
reaction.
Now, if the reaction is reversible or if the products are
readily inter convertible , then the
product percentage depends on their thermodynamic stabilities
under the similar reaction
condition.
Thus, if the amount of different products are determined by their relative thermodynamics stabilities, it is said to be thermodynamically controlled product.
Thus, if the amount of different products are determined by their relative thermodynamics stabilities, it is said to be thermodynamically controlled product.
For example , sulphonation of naphthalene at 160ᵒC is thermodynamically controlled.
When naphthalene is treated with concentrated H2SO4 at 353K, the main
product is 1-naphthalene sulphonic acid
whereas at 433K, the main product is 2-naphthalene sulphonic acid.
Further, when heated with concentrated H2SO4 , the 1- acid is converted into the 2-acid. Thus , the former is the kinetically controlled and the latter is thermodynamically controlled product.
The possible explanation for their relative stabilities is as follows , in 1-acid a steric repulsion between the hydrogen atom in 8-position and - SO3H group in 1-position makes the compound unstable at 353K temperature which is absent in 2-acid.
Thus , the former is the kinetically controlled and the latter is thermodynamically controlled product.
Since 1-acid is the kinetically controlled product, then K1 > K2 and therefore E2 >E1 . Also ,since 2-acid is the is thermodynamically controlled product, ΔH2 > ΔH1 .
The energy profile diagram of the sulphonation of naphthalene are as follows,
What is kinetically controlled product ?
When a chemical reaction gives rise to two or more products
,the nature of the products and their proportion in the products mixture
depends on the energetic of the reaction, that is, on the kinetics and the thermodynamic of the reaction.
Generally, it has been found that the faster a product is formed , the larger is its proportion in the product mixture.
Generally, it has been found that the faster a product is formed , the larger is its proportion in the product mixture.
When the proportion of a particular product in the final products mixture are determined from the rate of formation of that product, then the product is said to be a kinetically controlled product
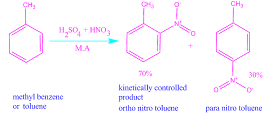
For example, nitration of toluene is kinetically controlled.
When toluene is treated with concentrated H2SO4 and
HNO3 it gives a mixture of o-nitro toluene ( 70% ) and p-nitro toluene ( 30%) .
Consequently, o-nitro toluene is the
main product. Although it is less stable
than p-nitro toluene.
Since the percentage of o-nitro toluene in the product mixture depends on the rate of formation , so it is kinetically controlled product.The energy profile diagram of nitration of toluene are as follows,
Since the percentage of o-nitro toluene in the product mixture depends on the rate of formation , so it is kinetically controlled product.The energy profile diagram of nitration of toluene are as follows,
Practice problems
- What is kinetically controlled product ?
- What is thermodynamically and kineticallycontrolled product ?
- Why sulphonation of naphthalene gives different products at different temperature?




No comments:
Post a Comment