What is homotopic ?
Two atoms or groups in a molecule are homotopic if the separate replacement of each group or atom
by some other group or atom produce the same molecule.
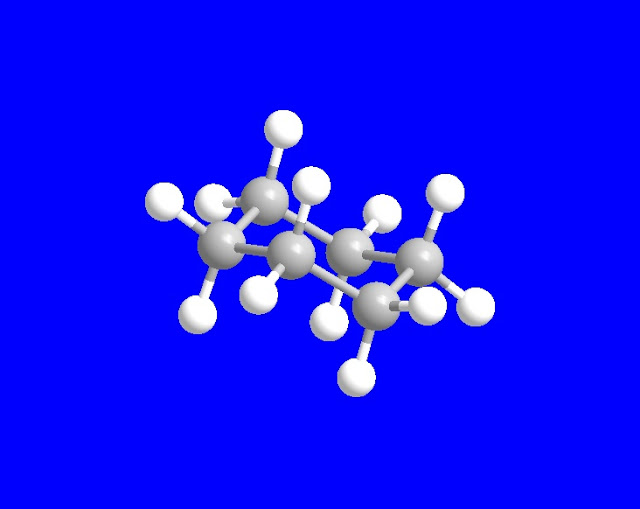
For example, the α-axial , β-axial and β-equitorial, α-equitorial H-atoms in chair conformation of cyclohexane
are homotopic . All the hydrogen atoms in methane are alike, so they are also called homotopic atoms.
Chair
conformation of cyclo-hexane in 3D form .
 |
What is diastereotopic ?
What is diastereotopic ?
Two atoms or groups in a molecule are diastereotopic if
replacement of each in turn by some other groups leads to a pair of
diastereomers.
For example, bromination of 3-methyl pentanoic acid gives a
pair of diastereomers.
The two α-H in 3-methyl pentanoic acid are diastereotopic.
What is enantiotopic ?
Two atoms or groups in a molecule are enantiotopic if replacement of each in turn by some other
group leads to a pair of enantiomers.
When optically active compounds are prepared by synthetic
methods, the usual results is a racemic modification.
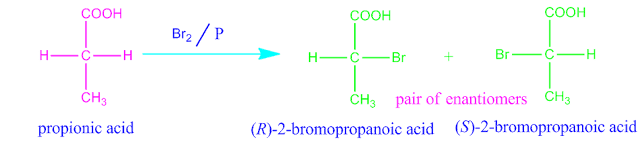
For example, bromination of propionic acid gives DL α-bromopropionic acid.
Although, the two H-atoms in propionic acid are
alike, yet replacement of one or other hydrogen does not produce same molecule,
a pair of enantiomers is produced. This two H-atoms are therefore said to be
enantiotopic.
Addition of HCN to acetaldehyde is also an example of
enantiotopic.
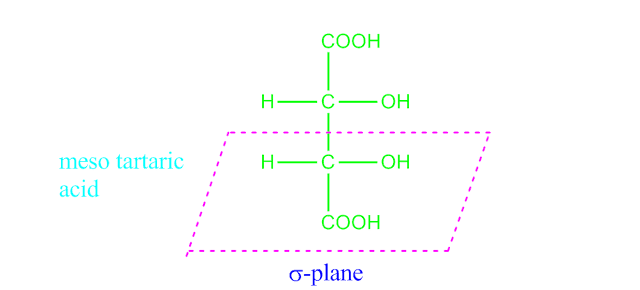
Why meso tartaric acid is optically inactive?
Meso tartaric acid contains two chiral carbon atoms, yet it is optically inactive. This is due to the
fact that meso tartaric acid has a plane of symmetry.
Practice summary:
What is homotopic ? Give example .









No comments:
Post a Comment