What is sodium hydrogen carbonate?
Sodium
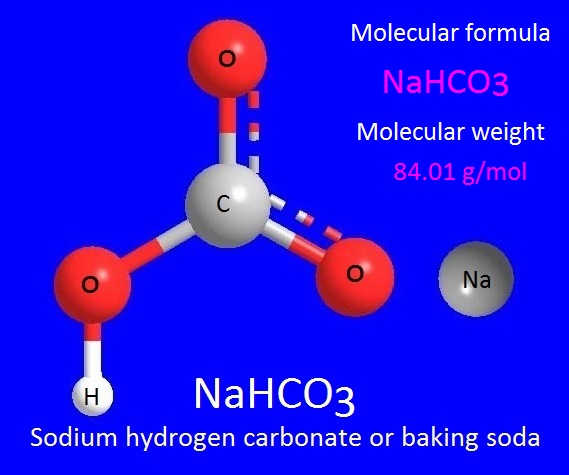
hydrogen carbonate is also name as sodium bicarbonate, is a
chemical compound with molecular formula, NaHCO3. It is also known as an acidic
salt or bi salt, which is made from carbonic acid.
Sodium hydrogen carbonate is commonly known as baking soda or bicarbonate of soda. It is a salt composed of a sodium cation (Na+) and a bicarbonate anion (HCO3-).
Sodium hydrogen carbonate is a white crystalline solid, but often appears as a fine powder. The molecular weight or molar mass of sodium hydrogen carbonate is 84.01g/mol. Sodium hydrogen carbonate salt is soluble in water. The density of sodium bicarbonate is 2.2 g/cc.
Uses of sodium hydrogen carbonate
Leavening agent in cooking
In cooking, baking soda is mainly used in baking as
a yeast agent. Baking soda causes
the batter to expand. For
this reason it is used to make cakes, quick bread, soda bread and other baked
and fried foods.
Baking powder
Baking
powder is also sold for cooking. It
contains about 30% bicarbonate and various acidic components. Many types of baking powders contain
sodium bicarbonate, including calcium acid phosphate, sodium aluminum phosphate
or tartar.
Pyrotechnics
Sodium bicarbonate is one of
the main ingredients in common "black snake" fireworks. When sodium
bicarbonate is heated CO2 and H2O are released. Both are also used to delay the
combustion reaction as they are flame retardant.
Used
as mild disinfectant
Sodium
hydrogen carbonate has weak disinfectant
properties.
For this reason it can be an
effective fungicide against some organisms. In agriculture, sodium bicarbonate is applied to the leaves to prevent fungal
growth.
Uses as fire extinguisher
Sodium bicarbonate is used to
extinguish electric fires. This is because heating sodium bicarbonate releases carbon dioxide.
This CO2 surrounds the fire. As a result, the fire
is extinguished due to lack of oxygen.
Medical uses and health
Sodium bicarbonate suspension
is used as an antacid to treat indigestion and heart burn. It reacts with HCl
acid in the stomach to produce salt, water and carbon dioxide.
NaHCO3 + HCl → NaCl + CO2 +
H2O
Sodium bicarbonate or baking
soda is used in cardiopulmonary resuscitation under medical supervision. It is usually used when the blood pH
is markedly low.
Again,
HCO3−ion is used to treat
hyperkalemia. This is because it will bring K + back to the cell
during acidosis.
Since baking soda or sodium bicarbonate can cause alkalosis, it is sometimes used to treat overdose of aspirin. It can be applied topically with three parts baking soda, one part water, as a paste to relieve bites and stings of some insects.
Personal hygiene
Sodium
bicarbonate is also used as an ingredient in some mouthwashes. It works as a
mechanical cleanser on the teeth and gums, neutralizes the production of acid
in the mouth, and also acts as an antiseptic to help prevent infections. It is
used in eye hygiene to treat blepharitis.
Cleaning agent
Sodium bicarbonate is used
for color and corrosion removal. Baking soda is usually added to the washing
machine to replace the water softener and remove odors from the fabric. It is
also as effective as sodium hydroxide in removing heavy tea and coffee stains
from cups.
Other uses of NaHCO3
Sodium bicarbonate is used as a cattle feeds
supplement, in particular as a buffering agent for the rumen. It is also used
as a neutralization agent in laboratory.
- What is sodium hydrogen carbonate?
- What are the uses of sodium hydrogen carbonate?
sodium
hydrogen carbonate, sodium bicarbonate, baking soda, bicarbonate soda, NaHCO3,
use of sodium hydrogen carbonate,
Read more: Catenation property of carbon


No comments:
Post a Comment