What is oxidation of ethene in organic chemistry?
Oxidation
of ethene or ethylene is an important chemical reaction in organic chemistry.
Because, on oxidation of ethene produces ethylene oxide which is very important
synthetic reagent.
There
are different methods for oxidation of ethene. The various methods for
oxidation of ethene are discussed below individually.
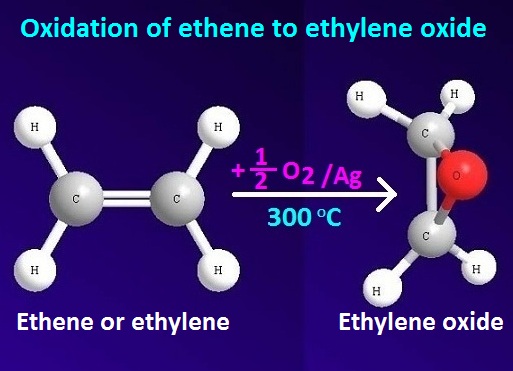
Oxidation of ethene by oxygen to ethylene oxide or epoxide
Oxidation
of ethene takes place in presence of oxygen by using silver catalyst at 300ᵒC temperature. The resulting product is
known as epoxy alkane or epoxide.
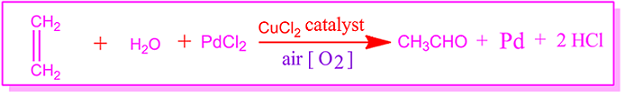
Oxidation of ethene to acetaldehyde by Wacker process
Again in the presence of air, when ethylene gas is passed through a slightly acidic PdCl2 solution mixed with CuCl2, ethylene is oxidized by oxygen in the air and converted to acetaldehyde.
This process for the preparation of acetaldehyde from ethene is called Wacker process.
Oxidation of ethene to ethylene glycol by KMnO4
The
oxidation of ethene can also happen in presence of alkaline KMnO4. It is very
important method. Potassium permanganate is a strong oxidizing agent.
There
are two types of oxidation of ethene may occurs in alkaline KMnO4 depending on
the reaction condition.
Oxidation
of ethene by cool dilute alkaline KMnO4 solution
Ethylene
is easily oxidized by cool dilute alkaline KMnO4 solution to produce ethane-1, 2-diol
or ethylene glycol. KMnO4 reduces itself into
MnO2.
Oxidation
of ethene by warm and concentrated alkaline KMnO4 solution
When
ethylene is heated by warm and concentrated alkaline KMnO4, the C = C bond of ethylene is broken. This
results in the break down of ethylene to produce formic acid.
The
producing formic acid is further re-oxidized by KMnO4 to produce carbon dioxide
and water.
Besides
these, oxidation of ethene or ethylene may also happen by using per acids, such
per benzoic acid, peroxytrifluoroacetic acid etc. Although, per acids is used
for oxidation of higher alkene.
Ethylene oxide reactions in organic chemistry
Ethylene
oxide is also known as oxiran. It is very important organic synthetic reagent.
A lot of ethylene oxide reactions are found in organic synthesis.
For
example, ethylene oxide reactions with water, amine, ammonia, Grignard reagent,
alcohol, hydrogen bromide, HCl, HCN etc are known. Some ethylene oxide
reactions are shown below.
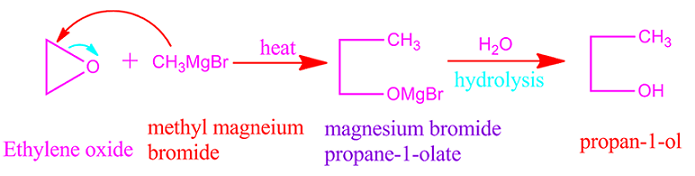
Ethylene oxide reaction with Grignard reagent
Ethylene
oxide reaction with Grignard reagent followed hydrolysis produce primary
alcohol with high molecular weight.
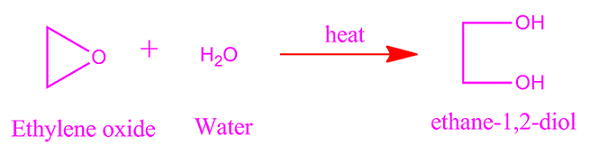
Ethylene
oxide reaction with water
Reaction
of ethylene oxide with water gives ethylene glycol. The reaction takes place in
presence of hydrochloric acid.
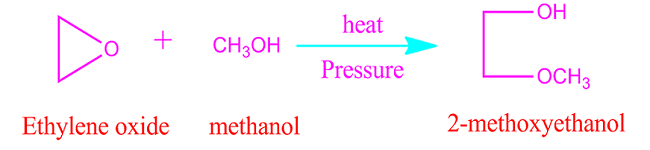
Ethylene
oxide reaction with alcohol
When
ethylene oxide reacts with alcohol then it form alkoxy alcohol. For example,
with methanol ethylene oxide produce 2-methoxy ethanol.
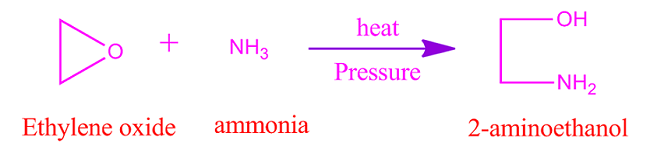
Ethylene
oxide reaction with ammonia
When
ethylene oxide reacts with ammonia then it form amino alcohol. For example,
with ammonia ethylene oxide produce 2-amino ethanol.
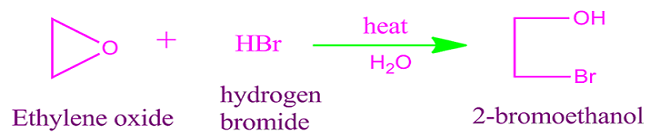
Ethylene
oxide reaction with hydrogen bromide
When
ethylene oxide reacts with hydrogen bromide then it form bromo alcohol. For
example, with hydrogen bromide ethylene oxide produce 2-bromo ethanol.
- What is oxidation of ethene in organic chemistry?
- Oxidation of ethene to acetaldehyde by Wacker process
- Oxidation of ethene to ethylene glycol by KMnO4
- Ethylene oxide reactions in organic chemistry
- Ethylene oxide reaction with Grignard reagent
- Ethylene oxide reaction with water
- Ethylene oxide reaction with alcohol
- Ethylene oxide reaction with amine
- Ethylene oxide reaction with ammonia
- Ethylene oxide reaction with hydrogen bromide
Oxidation of ethene, oxidation of ethene to acetaldehyde by Wacker process, oxidation of ethene to ethylene glycol by KMnO4, ethylene oxide reactions, ethylene oxide reaction with Grignard reagent, ethylene oxide reaction with water,
Read more : What is hydrolysis in organic chemistry




No comments:
Post a Comment